environmental
Applications
note
RestekCorporation • (800) 356-1688 • (814)353-1300 •
#59150
environmental
OptimizingMassachusettsVolatilePetroleumHydrocarbonGCAnalysis
TotalPetroleumHydrocarbon (TPH) analysisallows thecharacter-
izationofdozensof commerciallyavailablepetroleumproducts,
whichare themost prevalent environmental pollutants.The two
fractionsofTPH—volatilegasoline rangeorganic (GRO) com-
pounds, alsocalledpetroleumvolatileorganiccompounds
(PVOC), and the semivolatilediesel rangeorganic (DRO) com-
pounds—areanalyzeddifferentlydependingon theirboilingpoint
ranges.
Typicalmethods for the identificationof gasolineuseearlyand
lateeluting compounds todetermineananalyticalwindow for
total gasolinequantitation. Then,GCanalysisusespattern recog-
nition, the specific ratioof peaks thatmakeupaparticular com-
pound, to identify a fuel. If apattern fallswithin thiswindow, it
maybe reportedasgasolineandquantified. Difficultmatrices can
result inmisidentificationor poor quantitationof the sample, and
environmentaldegradation (i.e.,weathering) furthercomplicates
this analysis.
On January1, 1998, theMassachusettsDepartment ofEnviron-
mentalProtection (MADEP)promulgatedanewmethod, known
asVolatilePetroleumHydrocarbons (VPH) tobetter quantify
gasolines.Thismethod identifiesandevaluatesPVOCsbydiffer-
entiating and characterizing thearomaticand aliphatic fractionsof
gasolineusingaphoto-ionizationdetector (PID) anda flame ion-
izationdetector (FID) in series.Thedatagenerated from this
methodwill aid inevaluatinghumanhealthhazards thatmay result
fromexposure toPVOCs.Other states in theUSandprovinces in
Canadahaveadopted theVPHmethod for use in remediation, site
characterization, and toxicitydata (mixtures for othermethods are
listed in theUSTProduct Listing, lit. cat. #59617-A).
Difficultieswith theAnalysis
A largeproportionofVPH samples are soil.The soil isweighed in
the fieldandanequal amount ofmethanol is addedat the timeof
sampling. 100uLofmethanol extract is added to4.9mLofwater
and then is purged.Oneproblemwith this analysis is thatmost
purge-and-trapconcentratorswerenot designed tohave large
amountsofmethanol purgedonto their absorbent beds.The
VOCARB
™
3000andVOCARB
™
4000 trapshavedifficulty re-
taining
n
-pentaneand2-methylpentaneafter repeatedexposure to
methanol, causingpoor linearityof these compounds.We suggest
usingaBTEX trapbecauseof itsnon-polar properties.Youwill
experiencea slight decrease in response formethyl-
tert
-butyl-
ether, but itwill not compromiseyour detection limit.
ColumnSelection
All purge-and-trapmethods for this analysis result inbroad, early
elutingpeaks.Therefore, choosing the right chromatographic
column canprevent coelutionsandpoorquantitation. Many col-
umnsmaydrastically change thequantitationof aliphatic and
aromatic compounds, or suffer frompoor resolutionofmethanol
andmethyl-
tert
-butyl-ether or frompoor separationof pentane
and2-methylpentane. For optimized separationof light hydrocar-
bons and light gas additives, use theRtx
®
-502.2 column speci-
fied in theVPHmethod.
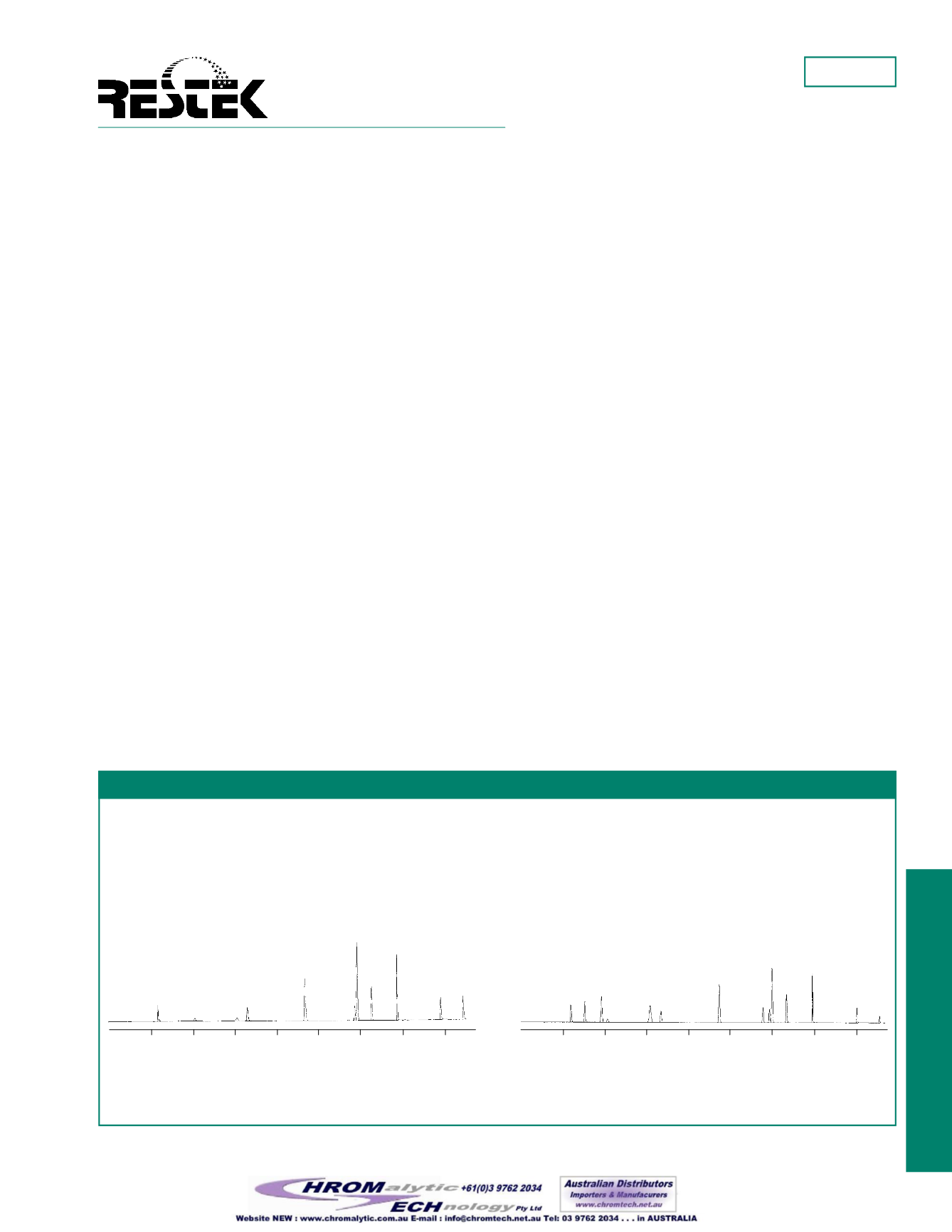
Figure1 shows the separationofVPHcompoundsonanRtx
®
-
502.2column, usingaPID (Figure1A) andanFID (Figure1B).
The first peak in thechromatogram ismethanol.Notice that it is
clearly separated from themethyl-
tert
-butyl-ether and
n
-pentane.
Figure1
To achieve optimumVPH analysis using anRtx
®
-502.2 column, usePID for aromatic compounds andFID for
aliphatic compounds.
Figure 1A: PID
105m, 0.53mm ID, 3.0µm (cat.# 10910).
Concentration:
on-column at levels listed;
Oven temp:
45°C to 90°C@ 3°C/min., to 140°@ 5°C/min.,
to 230°C@ 45°C/min. (hold 8min.);
Carrier gas:
helium@ 15mL/min.; Tekmar
®
Model LSC 2000;
Trap:
BTEX;
Purge:
helium@ 40mL/min.
for 11min.;
Dry purge:
2min.;
Desorb preheat:
245°C;
Desorb:
2min.@ 250°C;
Bake:
6min.@ 260°C.
Chromatograms courtesy of Severn Trent Laboratories, Burlington, VT.
1. methanol
2. methyl-
tert
-butyl-ether (60ng)
3. benzene (20ng)
4. toluene
5. ethylbenzene (20ng)
6.
p
+
m
-xylene (40ng ea.)
7.
o
-xylene (40ng)
8. 1,2,4-trimethylbenzene (40ng)
1. methanol
2.
n
-pentane (40ng)
3. 2-methylpentane (60ng)
4. methyl-
tert
-butyl-ether (60ng)
5. 2,2,4-trimethylpentane (60ng)
6. benzene (20ng)
7. toluene
8.
n
-nonane
9. ethylbenzene (20ng)
10.
p
+
m
-xylene (40ng ea.)
11.
o
-xylene (40ng)
12. 1,2,4-trimethylbenzene (40ng)
13. naphthalene (40ng)
14. 2,5-dibromotoluene (ss)
9. naphthalene (40ng)
10. 2,5-dibromotoluene (ss)
Figure 1B: FID
min. 4
8
12
16
20
24
28
32
1
7
2 3
4
5 6
89
10
11
12
13
14
1
7
2
3
4
5
6
8
9 10
min. 4
8
12
16
20
24
28
32


