pharmaceutical
Applications
note
Restek Corporation •
#59107
✸
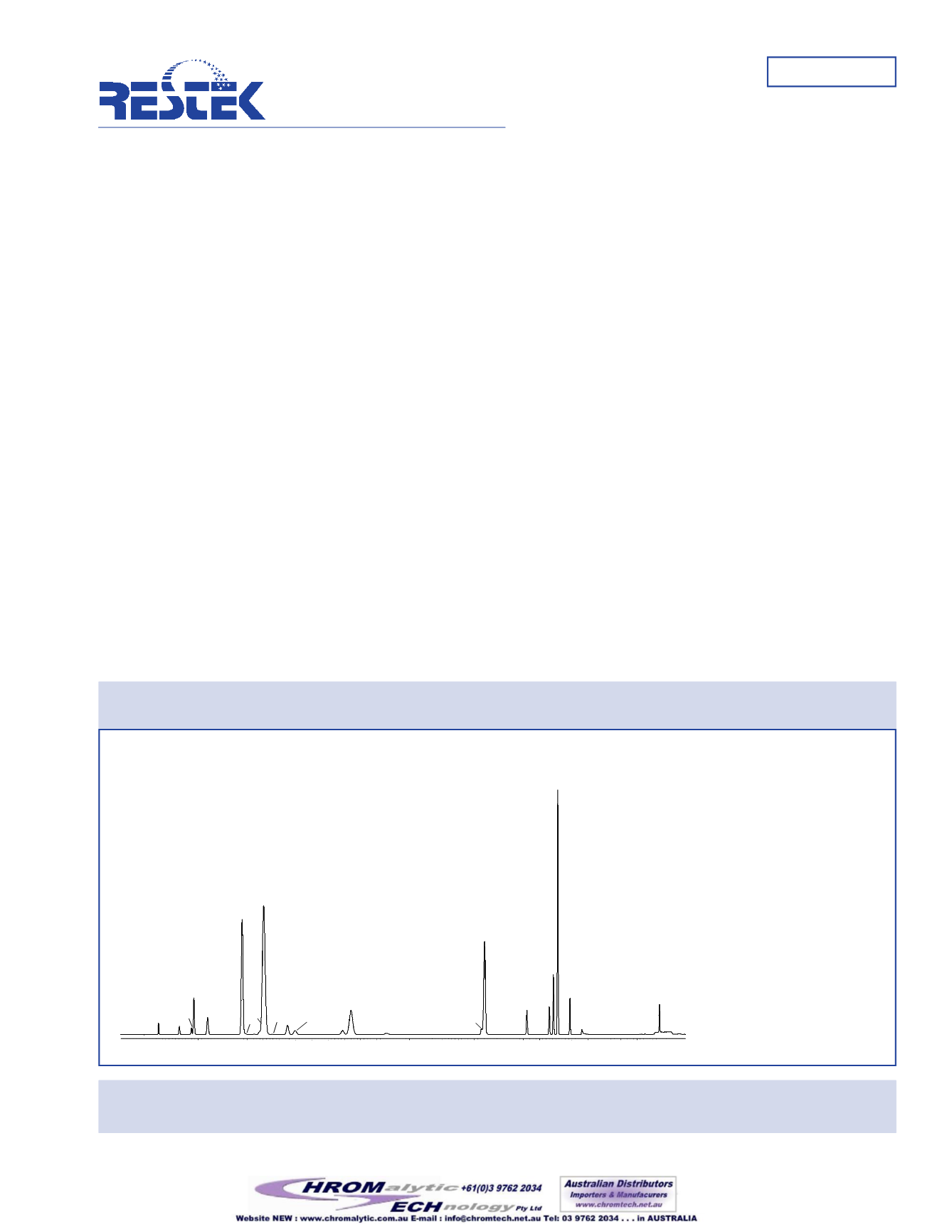
Figure 1:
TheRtx
®
-1301 column shows excellent resolutionofmost EuropeanPharmacopoeiaClass 1andClass 2
compounds at the regulation limit concentration.
EuropeanPharmacopoeiaTests—NewlyRevised for Residual Solvents
Residual solvent testing in pharmaceutical formulations can be
confusing. The International Conference onHarmonization
(ICH) has proposed a set of guidelines thatmay end the
confusion and theEuropeanPharmacopoeia (EP)was the first to
revise their regulations for clarity.
(1,2)
However, these guidelines
are challenging, containing over 60 compounds of regulatory
interest tomanufacturers of active substances, excipients, and
medicinal products. TheEPmethods also allow testing limits
based on either a concentration limit in a product, or calculated
from themaximum daily dosage of the product and the permis-
sible daily exposure limit of the solvent. These technical
challengeswill affect the samplingmethod and capillary column
needed to ensure precise and accurate results.
The twomost common sampling techniques for residual solvent
testing are direct injection and static headspace sampling.While
theEPmethod lists only static headspace sampling, the ICH
allows the use of any validated samplingmethod.Although the
majority of the regulated compoundsmay be successfully tested
by either samplingmethod, six of theClass 2 compounds cannot
be tested by headspace. These compounds—formamide,
2-methoxyethanol, N-methylpyrrolidone, sulfolane,
2-ethoxyethanol, and ethylene glycol—are available from
Restek in a separatemix at the regulatory limit for EP residual
solvent testing to be analyzed by direct injection.
Restek sells EP calibrationmixes at the regulatory concentration
limit, allowing the same sample:dilutant (1:20) ratio to be used
for the calibrationmaterial without any further concentration
correction back to the sample concentration.
See the product
listing forClass 1 andClass 2 residual solvent classifications.
Restek sells theClass 1 andClass 2 compounds in amix of
water:dimethylsulfoxide (90:10). The use of co-solvents helps
precision by limiting the loss of volatile analytes during standard
preparation and handling, and product dispersion during sample
preparation. Restek can provide custom analytical reference
materials inN,N-dimethylformamide (DMF) or 1,3 dimethyl-2-
imidazolidinone (DMI) tomeet certainEP testing requirements.
The recommended capillary columns for EP residual solvent
testing are theRtx
®
-1301 andStabilwax
®
.We can recommend
other columns for customers analyzing abbreviated residual
solvent lists. TheRtx
®
-1301 column shows excellent resolution
ofmost EuropeanPharmacopoeiaClass 1 and 2 compounds at
the regulation limit concentration (
Figure 1
). The Stabilwax
®
columnmakes an excellent confirmation column for the analysis
of residual solvents (
Figure 2
). Please call uswith your specific
compound list and our technical representativeswill find the
best column for your solvent list and provide youwith a price
30mx .53mm IDx3.0mmRtx
®
-1301 (cat.#16085-126)
Headspace injection of 28Class 1 and 2 residual solvents for
pharmaceutical processing. Prepared at the regulatory limit
concentration. Samples shaken and heated at
80°C for 15minutes, 1mL headspace injection.
Oven temp.:
40°C (hold20min.) to240°C@10°C/min.
(hold20min.)
Inj./det. temp.:
200°C/250°C
FID sensitivity:
1.1x10
-11
AFS
Carrier gas:
hydrogen@35cm/sec.
Split ratio:
2:1
1. methanol
2. 1,1-dichloroethene
3. acetonitrile
4. methylene chloride (dichloromethane)
5. hexane (C6)
6. cis-1,2-dichloroethene
7. nitromethane
8. chloroform
9. cyclohexane
10. 1,1,1-trichloroethane
11. carbon tetrachloride
12. benzene
13. 1,2-dimethoxyethane
14. 1,2-dichloroethane
Toplaceanorder:
call 814-353-1300or
800-356-1688, ext. 3.
15. trichloroethylene (1,1,2-trichlorethene)
16. methylcyclohexane
17. 1,4-dioxane
18. pyridine
19. toluene
20. 2-hexanone
21. chlorobenzene
22. DMF
23. ethylbenzene
24.
m
-xylene
25.
p
-xylene
26.
o
-xylene
27. N,N-dimethylacetamide
28. 1,2,3,4-tetrahydronaphthalene
1
2
4
5
6
9,10
12
13,14
15
16
18
19
20
21 22,23
26
27
28
3
8
7
11
17
2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34
24,25
References:
1. “ICHHarmonizedTripartiteGuideline, Impurities: Guideline for Residual Solvents,” The Fourth International Conference onHarmonization, July 17, 1997.
2. EuropeanPharmacopoeiaSupplement, January1999, pp.14-15, 208.
quotation for the custom analytical referencematerials you need.


