Sulfur Trapping at lowPPB levels using theSRI
8610CGasChromatograph
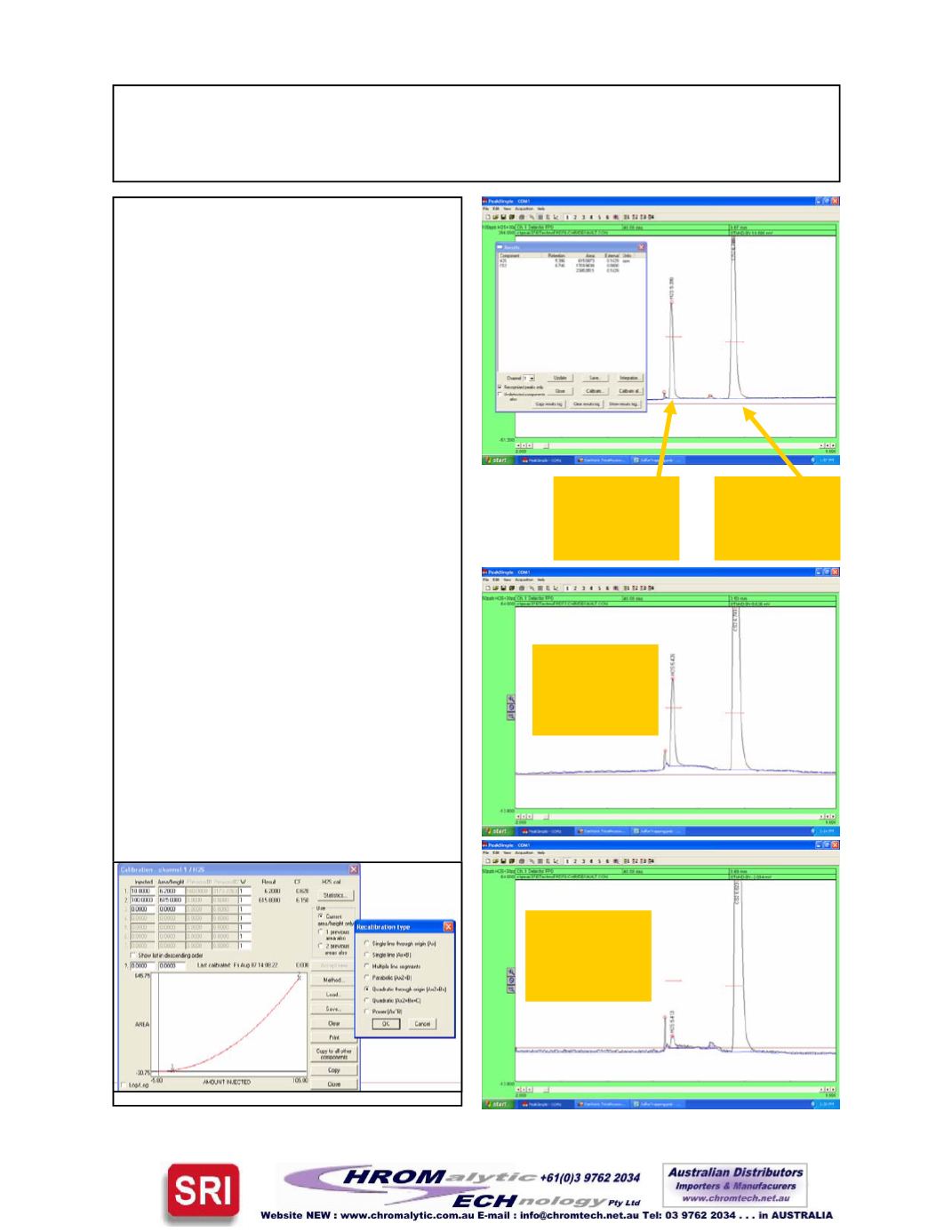
The top chromatogram showsH2S
at 100ppb andCS2 at 30ppb.
TheCS2 peak is bigger than the
H2S peak because theCS2 traps
better than theH2S and has two
sulfur atoms permolecule rather
than one. In general, the higher
the sulfurmolecule’s boiling point,
the better it traps.
The second chromatogram shows
H2S at 50ppb. Note that the area
is 1/4th as big as the area for the
100ppb peak. This is normal be-
cause theFPD detector responds
to the square of the sulfur concen-
tration, so half the amount injected
produces 1/4 the area. The cali-
bration curve is quadratic as
shown below.
The third chromatogram shows
10ppbH2S againwith 30ppbCS2.
Page 8
30ppbCS2
Peak
Area=1769
100ppbH2S
Peak
area=615
50ppbH2S
Peak
area=168
10ppbH2S
Peak
area=19


