9
GeneralGuidelines for Interpreting theTrends
●
You should use the performance charts as a guideline
when choosing an adsorbent.
●
We list the analytes by their retention order from an
SPB-1 capillary column. They are in the order of their
boiling point, with the exception of Acrylonitrile and
1,2-Dichloroethane. (
See Table 2
)
●
The adsorbents were desorbed at their maximum
desorption temperature. (
See Table 1
)
●
You should consider the effects of water when choosing
an adsorbent, sincewe based this research on the
challenge of dry nitrogen.
Observing theTrendLeft toRight - Across theRows:
(Increased volume per analyte)
Starting at the 0.2-Liter volume, looking at one analyte:
1. If the row is solidGreenacross all six volumes— then this
adsorbent is a good choice for this analyte.
2. If the row startsGreenand changes toYellowand/or Red,
then the analyte is breaking through the adsorbent. Note:
Whensampling,maintainasamplevolumewithin thegreen
limits.
3. If the row is Yellow or Red –Choose another adsorbent.
Observing theTrendTop toBottom - Down theColumns:
(Increased Boiling-point per analyte)
Starting at the 0.2-Liter volume, looking at one volume:
If thechart isgreenat the topandchanges toYellow,and/orRed–
then theadsorbent is capableof efficiently retainingand releas-
ing theanalyteswith lowboilingpoints.As theboilingpoint of the
analytes increase, they become too strongly adsorbed (as indi-
cated by the * symbol or are irreversibly adsorbed). The
Carboxen(s) are a good example of this trend). Always place a
weakerbedofadsorbent in frontof this typeofadsorbent tokeep
these analytes from reaching this adsorbent.
If thechart isRedand/orYellowat the topandchanges toGreen–
then theadsorbent is capableof efficiently retainingand releas-
ing theanalyteswithhigher boilingpoints. As theboilingpoint of
the analytes decrease, they begin to break-through the adsor-
bent. The Carbopack(s) and Porous Polymers are a good ex-
ampleof this trend. Placea stronger adsorbent behind this type
of adsorbent to retain and release the low boilers.
Using theCharts toDesignaMulti-BedTube
You can use the data from the charts to construct a multi-bed
adsorbent tube.As thedata illustrates there isnooneadsorbent
thatwillboth retainand release theentire listofanalytes.Youcan
construct amulti-bed tubebyplacingaweaker adsorbent at the
inlet followedbyastrongeradsorbent.Youcancreate two, three
and fourbed tubes.Youcan tailor theadsorbentconfiguration for
the sampling application. The Carboxen(s)/Carbosieve S-III
should always be used along with a weaker adsorbent if the
environment tobesampledcontainshigherboilingpointanalytes.
Youcanuseasingleormulti-bed tubepackedwithaCarbopack
or a Porous Polymer and not include Carboxen(s)/Carbosieve,
allowing the low boiling analytes to pass through the tube. For
example, inmany caseswhenusinga liquid standard, it isoften
desirable toallow thesolvent (i.e.Methanol) topass through the
adsorbent while the higher boiling point analytes are retained.
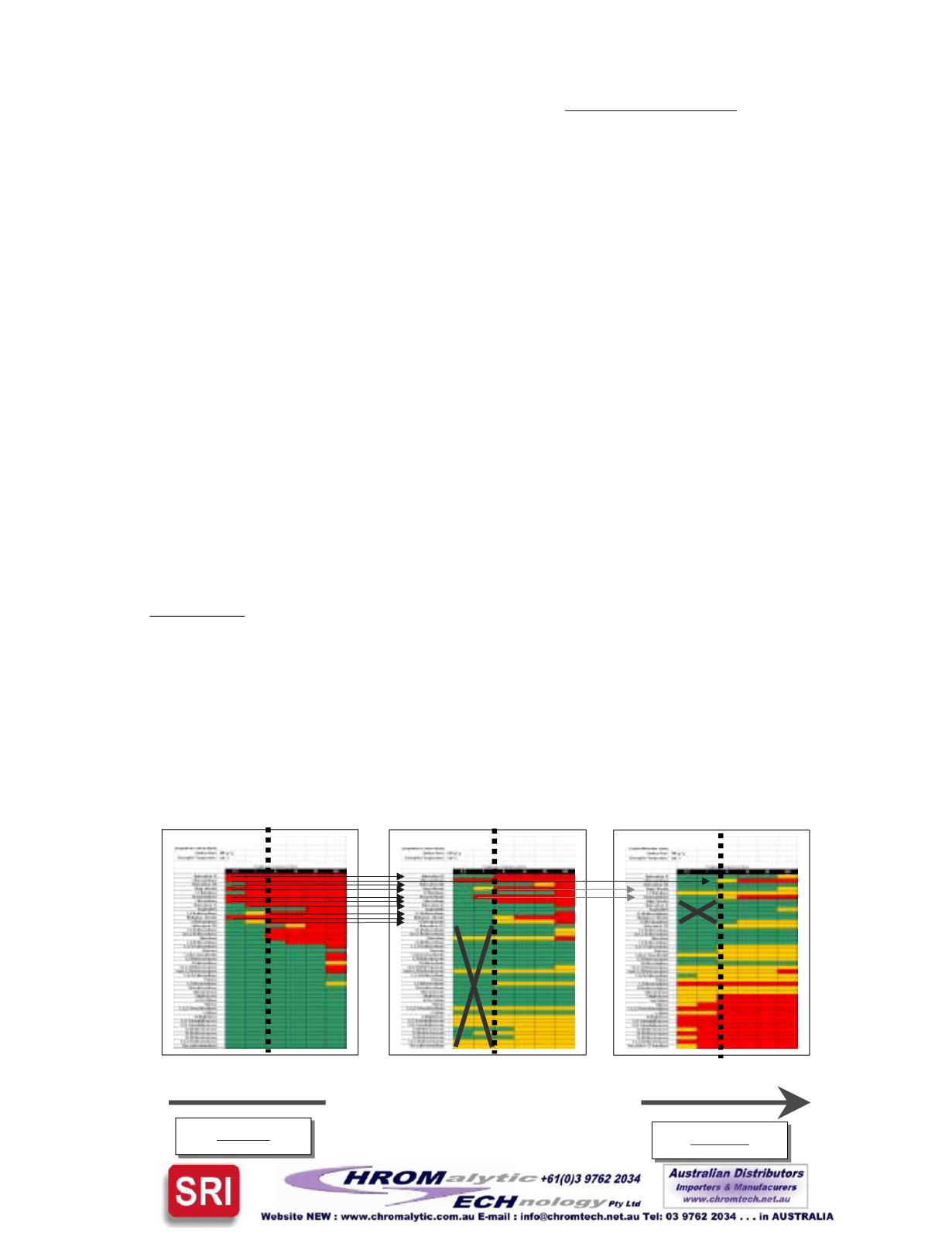
Theexamplebelow illustrates the trend to look forwhendesign-
ing a multi-bed tube. In this example, the goal is to choose a
combinationof threeadsorbents that can retain theentire list of
43 analytes for a sample volume up to a 1-Liter. The large gray
X(s) indicate those analytes that are retained by the absorbent
bed that precede it. The black arrows illustrate those analytes
that break-through the first bed, and are then retained by the
second bed.
Note, one of the analytes (indicated by black)
actuallybreak-through thesecondbedand is retainedby the last
bed.
Thegrayarrows illustrate thoseanalytes thatbreak-through
the second bed and are retained by the third (last) bed. The
dotted black line denotes the 1-Liter volume
.
Weakest
Strongest
SamplingDirection
(In order of increasing adsorbent strength)
First Bed
SecondBed
ThirdBed
CarbopackB
CarbopackX
Carboxen-1018


