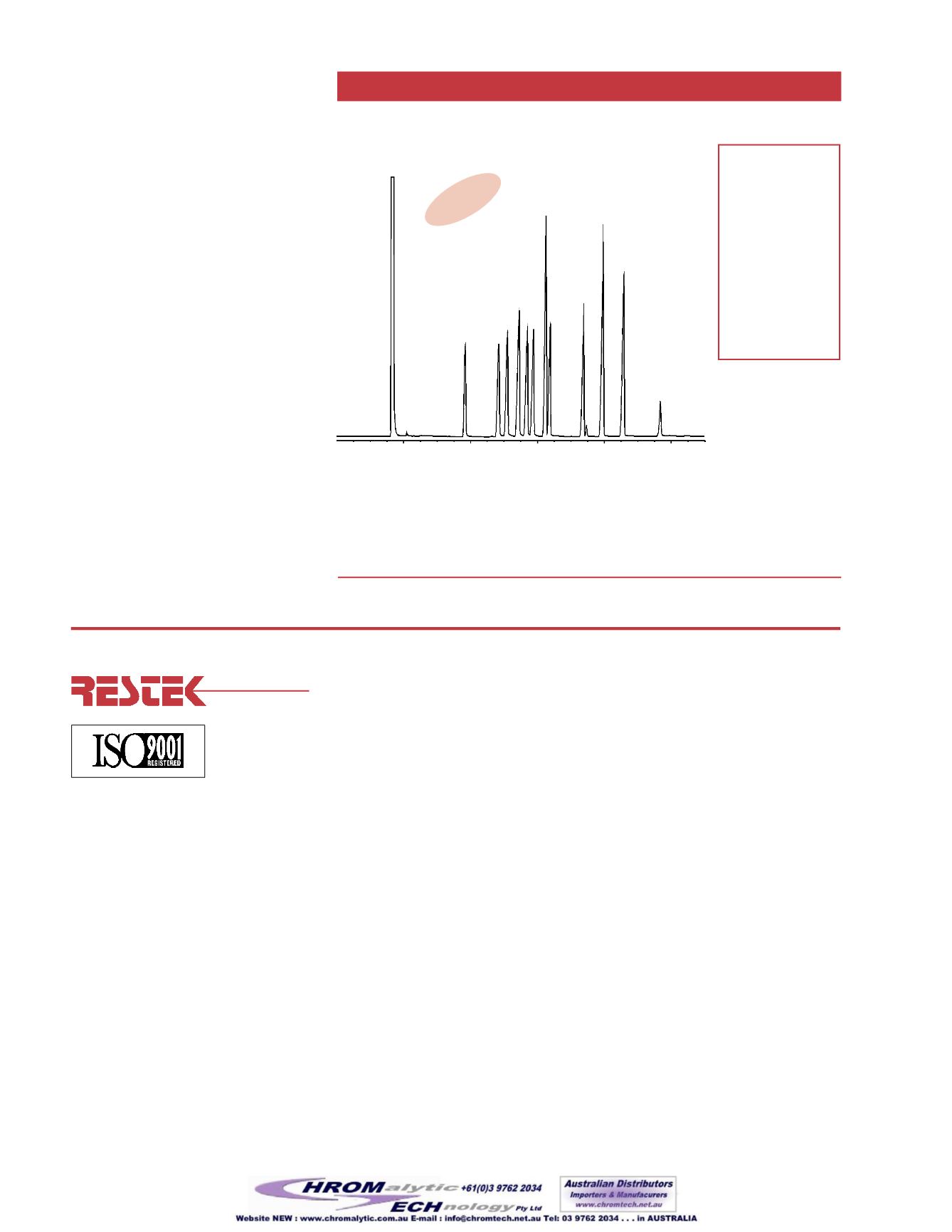
min. 2 4 6 8 10
Compounds
1. barbital
2. aprobarbital
3. butalbital
4. amobarbital
5. talbutal
6. pentobarbital
7. methohexital
8. secobarbital
9. thiopental
10. hexobarbital
11. mephobarbital
12. phenobarbital
30m, 0.32mm ID, 0.50µmRtx
®
-35 (cat.# 10439). 1.0µl split injection of barbiturates.
Oven temp.:
210°C (hold 2min.) to 300°C@ 7°C/min. (hold 2min.);
Inj./det. temp.:
300°C;
Carrier gas:
helium;
Linear velocity:
35cm/sec. set @ 210°C;
FID sensitivity:
5.12 x 10
-10
AFS
Split vent:
30:1
1
2
Figure 2
Underivatizedbarbiturates onanRtx
®
-35.
c-gram #3035
3
4
5
6
8
9
10
11
7
12
Optimized
usingPro ezGC
™
!
For permission to reproduce any portion of this application note, please contact Restek’s publications/graphics department by phone (ext. 2128) or FAX.
excess of 250°C inorder to efficiently
complete thederivatizationprocess.
Analysis of barbiturates can alsobe
performedonunderivatizedcompounds.
However, underivatizedbarbiturates
have a tendency toproduceoverloaded
or tailingpeaks.Maintain injectionport
liners, guard columns, and analytical
columns regularly to achievegoodpeak
shape and adequate resolution.
Figure2
shows the separationof a set of
underivatizedbarbiturates using an
Rtx
®
-35column. Lower polarity
stationaryphases like theRtx
®
-5 canbe
used to separate the barbiturates, but
intermediatepolarity stationaryphases
tend toprovidebetter peak shape and
improved resolution.
Barbiturates are an important part of
drug screening. Extra care shouldbe
takenwhen analyzingbarbiturates in
either thederivatizedor underivatized
form. Intermediatepolaritycolumns
combinedwithwellmaintained
injectionport liners andguard columns
will contribute tobetter peak shape and
resolution.


