environmental
Applications
note
Restek Corporation • (800)356-1688 • (814)353-1300 •
#59120
environmental
Rtx
®
-CLPesticides andRtx
®
-CLPesticides2Columns:The Ideal
Confirmational Pair for AnalyzingPolychlorinatedBiphenyls (PCBs)
Polychlorinatedbiphenyls (PCBs) are a groupof industrial
organochlorine chemicals that have become amajor environmental
concern. Since the 1950’s, over onemillionmetric tons of PCBs
have beenproduced.They areverypersistent in the environment
andbioaccumulate in living systems.All PCBs are practically
insoluble inwater, but they are soluble inhydrophobicmedia like
fats or oily substances. Theywere used commerciallybecause they
are chemically inert liquids and are difficult toburn; theyhave low
vapor pressures, are inexpensive tomake, and are excellent
electrical insulators.As a result, theywere used extensively as
coolant fluids in transformers and capacitors; and later as plasticiz-
ers, de-inking solvents, heat transfer fluids inmachinery, and
water-proofing agents, amongother uses.
Because of their persistence and their solubility in fatty tissue,
PCBs in food chains undergobiomagnification. Strongheatingof
PCBs in the presence of oxygen can lead to the formationof
polychlorodibenzofurans (PCDF),which are structurally and
toxicologically similar todioxins. Commercial PCBmixtures (e.g.,
Aroclor
®
mixtures) contain small amounts of PCDF as a result of
the synthesis. PCBmixtures are not highly toxic, but toxicitydue
to thePCDF concentrationhas caused concern.
CertainPCB congeners canbe highly toxic; toxicitydepends on
where the chlorine substitution resides on the biphenylmolecule.
The congenerswithout chlorine substitutionon the orthopositions
are themost toxic. These are termed “coplanar PCBs” because the
phenyl rings canmaintain a planar geometry to eachother. This
makes these compounds “dioxin-like,” and themost toxic of these
PCB congeners is one-tenth the toxicityof the 2,3,7,8-tetrachloro
dibenzodioxin. The coplanar PCBs alsohave been implicated as
endocrine disrupters.
1
It is important, therefore,whendesigning aPCB analysismethod
todetermine if the separationwill be by specific congener (for
toxicity) or by commercialAroclor
®
mixture. The commercial
synthesis of PCBs results in chlorinationof the biphenylmolecule,
and this reactionproduces amixture ofmanyof the 209 congeners
of thePCB family.
Namingof the specific congeners follows the positional numbering
shown inFigure1. Because the IUPACnames for these com-
pounds are long, the congeners are normally referred toby their
IUPACnumber orBZnumber as definedbyBallschmitter and
Zell.
2
The exact proportions of congeners in theAroclor
®
mixtures
depends on the ratioof chlorine tobiphenyl, the reaction time, and
the temperature.Althoughmanyof thePCB compounds are solids,
themixtures usually are liquids or low-melting-point solids.
Commercially, thePCB compoundswere not isolated. Instead,
theywere sold as partially separatedmixtures,with the average
chlorine content indifferent products ranging from21% to68%.
TheseAroclor
®
mixtures are, therefore, composedof a number of
individual PCB congeners andhave a characteristic profile
dependingon the percent of chlorine substitution. TheAroclor
®
mixtures arenamedby thenumber of carbons (12), followedby
theweight%of chlorine (42).ThusAroclor
®
1242 represents a
mixture of PCB compoundswith an averageweight percent of 42.
There are 9 commonAroclor
®
mixtures: 1221, 1232, 1242, 1248,
1254, 1260, 1262, 1268, and1016.Aroclor
®
1016does not follow
the same naming sequence, and appears chromatographically
similar to1242. Samples from contamination sites often are
quantitated and reported as concentrationof PCBs, i.e., as
Aroclor
®
mixtures. This analysis requires the individual PCB
Aroclor
®
mixtures tobe analyzed as standards, then the sample
extract chromatograms are compared to the standards toqualita-
tively identify theAroclor
®
mixtures. Once this identificationhas
beenmade, the quantitation canbe performedby selecting five of
the largest peaks and treating them as individual compounds, then
reporting the average concentration.
Due to the unreactive nature of thePCBs, instrument conditions
and column choice is less critical thanwhen analyzing chlorinated
pesticides.When choosing columns, it is important to select
stationaryphases that have lowbleed andhigh thermal stability;
allowing the columns tobe bakedout at the endof the run to
prevent carryover fromone injection to the next. Becausemany
instruments used for the analysis of PCBs alsomaybeused for
pesticide andherbicide analyses, the columnpair of choice is the
Rtx
®
-CLPesticides andRtx
®
-CLPesticides2 columns. This column
pair provides excellent separationof the pesticide andherbicide
compounds, lowbleed, high thermal stability, and they are
designed to compliment eachother for primary column analysis
and secondary column confirmation.
Figure 2 shows the chromatograms obtained for seven commercial
Aroclor
®
mixtures injectedon theRtx
®
-CLPesticides column.
Figure 3 shows the chromatograms for the samemixtures injected
on theRtx
®
-CLPesticides2 column.
Table 1 (onback) lists the retention times for the individual 209
PCB congeners on these same stationaryphases.The analysis of
PCBs by congener requires eachpeak tobe treated like an
individual component;making a standard curve for eachof the
congeners of interest.Whilemany laboratories are interested in the
analysis of PCBs by congener,most donot need, or desire, to
analyze all 209. For this reason, the retention table is listed, and
conditionsmaybemodified tobetter suit the particular separation
inyour laboratory. If you have questions regarding the analysis of
PCBs by congener, contact Restek’s technical service team at 800-
356-1688or 814-353-1300, ext. 4, or contact your local Restek
representative.
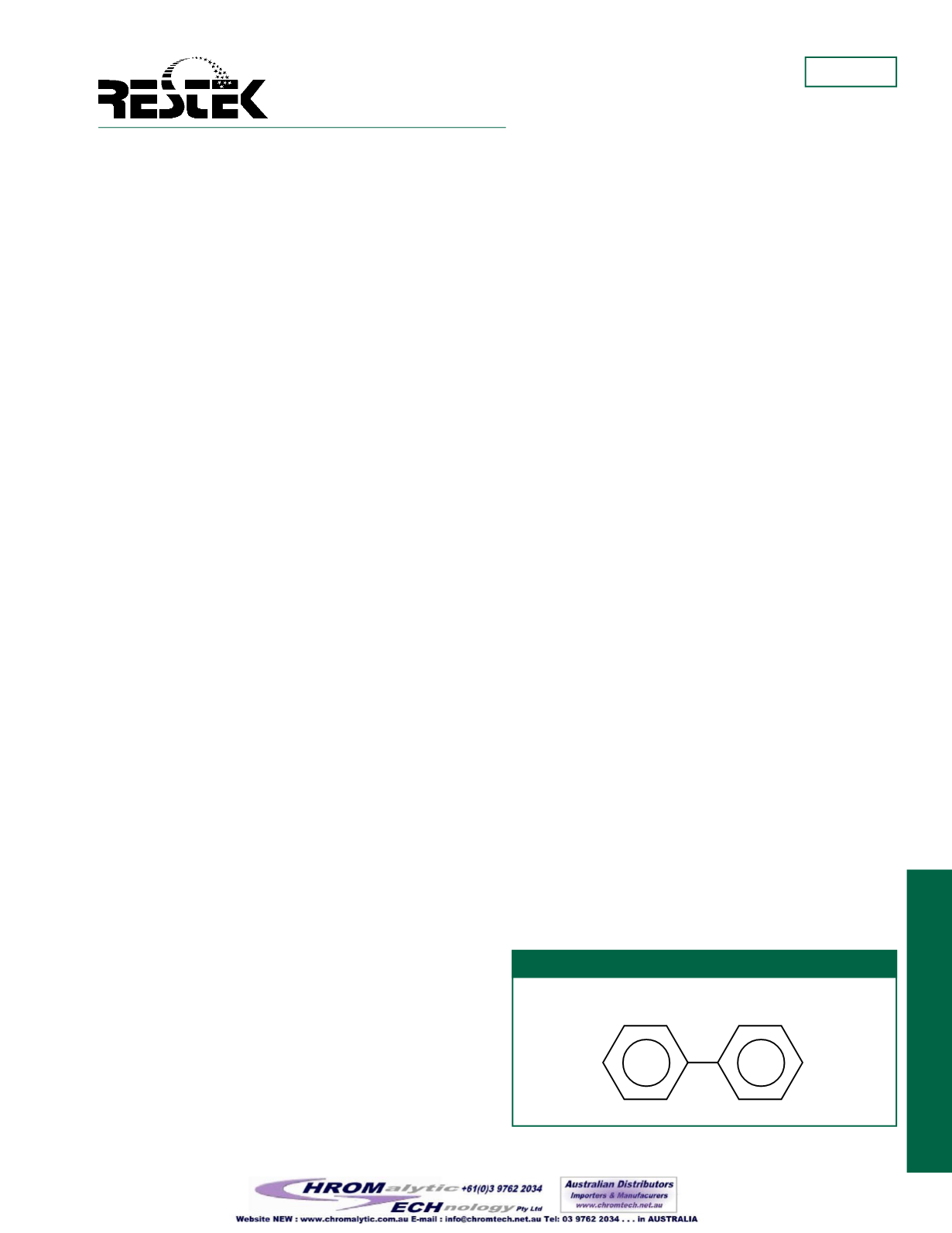
Figure1
Structure of polychlorinated biphenyls.
2
3
4
5
6
2'
3'
4'
5'
6'


