nutraceuticals
Applications
note
Restek Corporation • (800) 356-1688 • (814) 353-1300 •
#59364
nutraceuticals
AnalyzingNutraceutical Products by Liquid andGasChromatography
Introduction
The idea of using herbal compounds to enhance one’s health
has been around for thousands of years. Over the past decade,
however, the nutraceutical industry has seen rapid growth as
more people add flowers, leaves, roots, and fruits of botanicals
to their diets in the hopes of gaining health benefits. The
dietary supplement industry (including vitamins, minerals,
herbals, and amino acids) was approximately $16 billion in
2000with botanicals accounting for 25%.
1
Herbal ingredients
that oncewere packaged primarily in pills and capsules and
found in health food stores, now can be found in fruit juices,
spreads, and snack foods.
2
This includes ingredients such as
glucosamine, added to ease aches and pains; and kava or St.
John’swort, added for calmness and a sense of well-being.
Herbal products are very complex, often containing hundreds of
compounds, and it is not always clear which compounds are
responsible for the beneficial properties.Marker compounds—
phytochemicals that have been identified and are known to
have some relationship to the reported health benefit—can be
evaluated qualitatively to identify a rawmaterial or to verify
purity. To determine the concentration or strength of amaterial,
quantitative analysis is necessary. Other testing typically per-
formed on rawmaterials includes a physical exam, microscopy,
and determination of ash, heavymetals, residual fumigants, and
pesticide levels.Microbiological testing can be included
depending on the herbal material.
Dietary supplements are regulated under theDietary
Supplement HealthEducationAct (DSHEA) of 1994. Before
DSHEA, nutraceuticalswere regulated as either foods or drugs,
depending on their intended purpose.
3
The Food andDrug
Administration (FDA) has the authority under DSHEAto take
action against unsafe products or improperly labeled products.
For example, the FDAcan stop the distribution of products that
it finds to be toxic, unsanitary, that increase the risk of illness or
injury, or that make unsubstantiated health claims. Under cur-
rent regulations, however, FDApre-market approval of dietary
supplements is not required. This leaves the testing of dietary
supplements to the discretion of themanufacturer.
Several organizations have recognized a need for standardizing
procedures of herbal product analysis and are involved in pro-
grams that will assist the FDAin the regulation of the dietary
supplement industry. TheUSPharmacopoeia (USP) has
launched a dietary supplement certification pilot program to
address the issue of product quality. TheUSPprogram seeks to
ensure that the product contains the ingredients declared on the
label at the reported levels; that the product iswithin the
required limits on contaminants; and that the general require-
ments for themanufacturing practices of dietary supplements
are satisfied.
1
This program ismeant to complement DSHEA
and allows companies to add aUSPcertificationmark on their
label if the requirements aremet. The pilot program includes
post-market surveillance of the nutraceutical products and audit-
ing of themanufacturing facilities.As ofAugust 2001, theUSP
had over 20 official monographs for herbal materials, with
manymore in revision or draft form.
3
Other organizations, such as the Institute for Nutraceutical
Advancement (INA), areworking to develop newmethods for
the quantitation ofmarker compounds. The goal of the INA
MethodValidation Program (MVP) is to submit these new
methods to an organization (e.g.,Association of Official
Analytical Chemists [AOAC] International) for collaborative
study and inclusion in their official methods program.AOAC
International also has aDietary Supplement TaskGroup that
provides a standard set of procedures for the analysis of botani-
cal compounds.
High performance liquid chromatography (HPLC) and gas chro-
matography (GC) are excellent tools for quantitative analysis of
marker compounds in botanical samples. Thin layer chromatog-
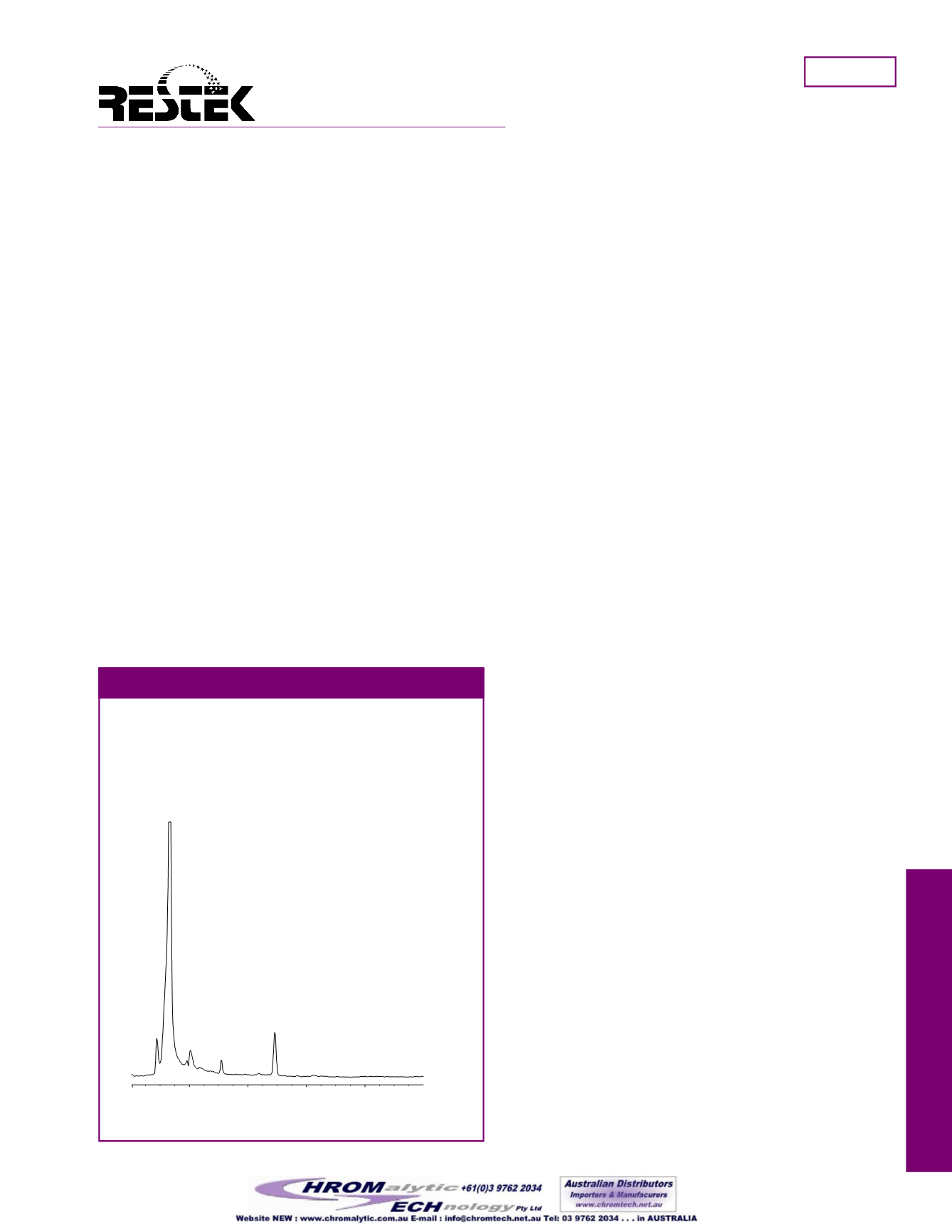
Rapidly analyze allicin in garlic byHPLC using a
Pinnacle II
™
C18 column.
Figure 1
0
2
4
6
8 min.
1
Column
Pinnacle II C18
Catalog #: 9214565
Dimensions: 150mm x 4.6mm
Particle size: 5µm
Pore size: 110Å
Conditions
Mobile phase: methanol:water (50:50)
Flow: 0.8mL/min.
Temp.: 30°C
Det.: UV@ 240nm
Sample
1.2mg/mL deodorized garlic tablet in
deionizedwater.
Inj.: 20µL
Peak list
1. allicin
LC_0174


