
 www.restek.com
www.restek.com
22
By Kevin A. Schug, Ph.D.
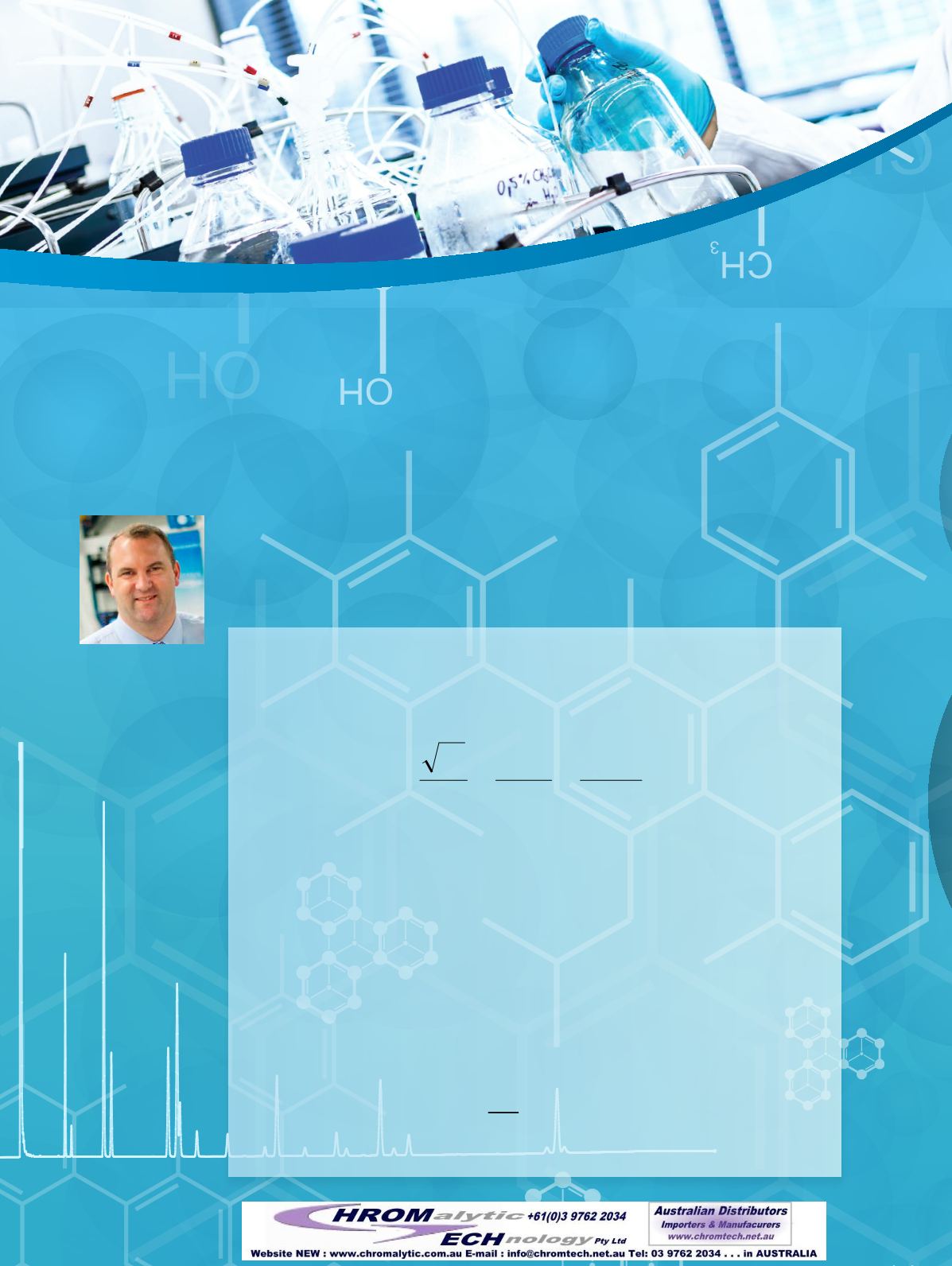
The name of the game in chromatography is the separation of chemical compounds. The resolu-
tion of one analyte from another in a chromatographic separation is determined by three main
factors: efficiency, selectivity, and retention. The interplay of these is described by the master
resolution equation,
where N is the number of theoretical plates (a measure of efficiency), α is selectivity, and k’ 2 is
the capacity factor (or retention factor) for the later eluting peak of the analyte pair of interest.
Incidentally, in some forms of the master resolution equation, an average capacity factor k’
avg
,
calculated from the retention of both analytes, is used in the third term. As we are largely con-
sidering a pair of closely eluting analytes, the difference between k’ 2 and k’
avg
would be minimal.
The magnitude of contributions of each of the three terms in Equation 1 to resolution varies, but
the maximization of each term (without the complete disregard of the other two) will help yield
the separation of analytes of interest (Rs ≥ 1.5 is the target value for baseline separation).
Here, we focus on the selectivity term. Selectivity is defined in Equation 2 as
The Role of Selectivity in Liquid Chromatography
Method Development
Dr. Schug is an Associate Professor and Shimadzu Distinguished Professor of Analytical Chemistry in
the Department of Chemistry and Biochemistry at The University of Texas at Arlington. He specializes
in the application of modern sample preparation, chromatography, and mass spectrometry techniques
for trace qualitative and quantitative determinations from complex mixtures. He is also active in drug
discovery, protein analysis, and environmental assessment.
Innovators in Chromatography
A continuing series of guest editorials contributed by collaborators and internationally recognized leaders in chromatography.
′+
′
−
=
2
2
1
1
4
k
k
N R
s
α
α
1
2
k
k
′
′
=
α
(1)
′+
′
−
=
2
2
1
1
4
k
k
N R
s
α
α
1
2
k
k
′
′
=
α
(2)











