
.....
V
Pharmaceutical
Beyond (18-lncrease Retention of Hydrophilic
Compounds Using Biphenyl Columns
----~--~-
Searching for a better way to reta in hydrophilic aromatic drug compounds?Biphenyl phases, such
as the
Pinnacle
™
DB Biphenyl
column, provide greater retenti on than alkyl phases. Use a
Biphenyl column to separate difficult-to-retain pola r aromatics from unretained matrix contaminants.
Many drug classes include com pounds with aromatic ring struc tures, some of
which also contain a sulfone or sulfoxide group. Both sulfur gro ups have dip ole
moments, adding a hydrophilic character to compounds containing th ese func
tion al groups. Th e analysis of hydrophilic com pounds on a traditional alkyl col
umn (e.g., C IS) can be prob lematic, since alkyl columns depend on hydrophobic
(dispersive) interactions for retention. Since the sulfone and sulfoxide groups con
tain
TC
bonds, the Biphenyl column's affinity toward compo unds contain ing these
bonds makes it a logical choice when increased retenti on of compounds contain
ing these gro ups is desired.
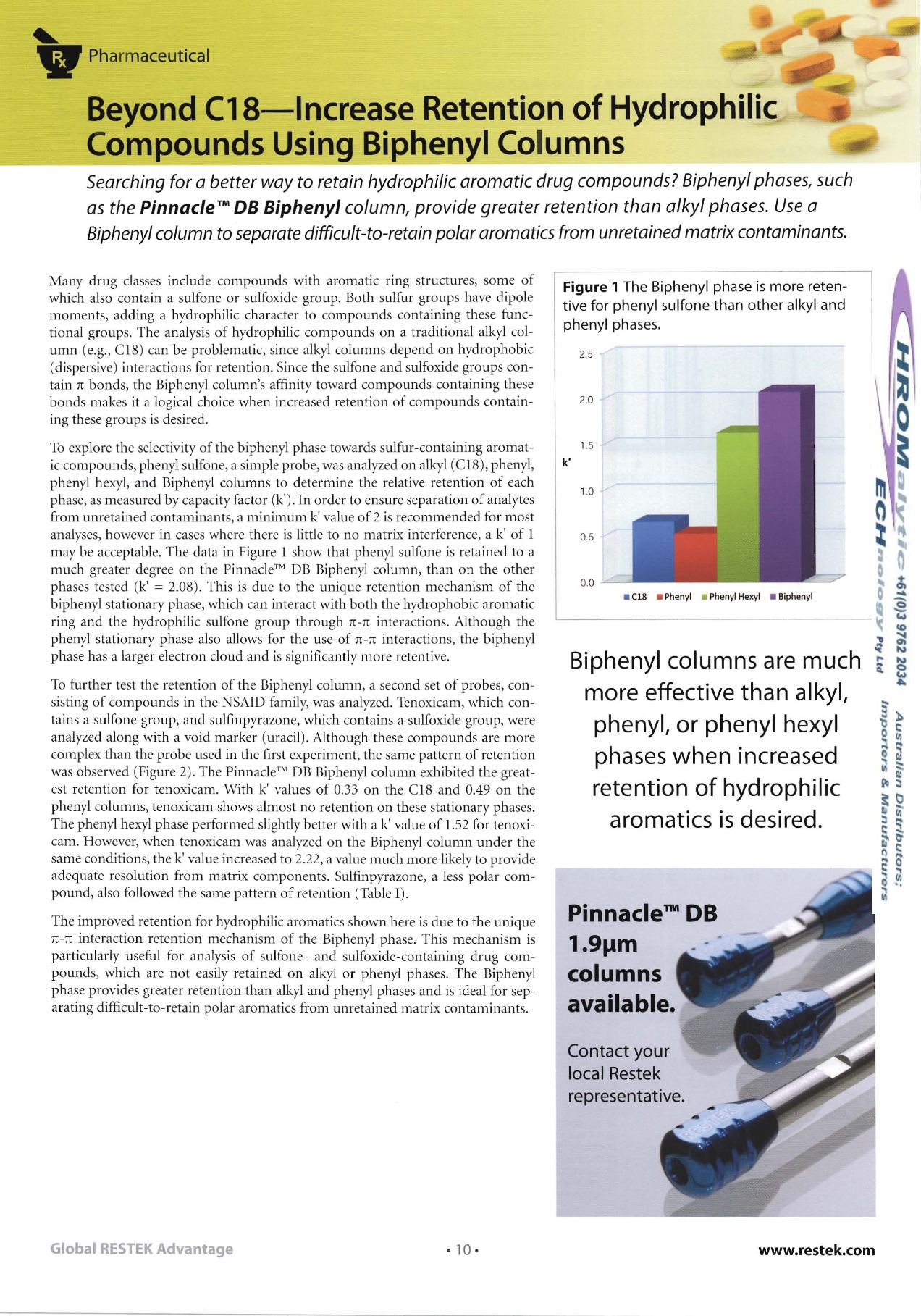
To explore the selectivity of th e biphenyl phase toward s sulfur-containing aromat
ic compounds, ph enyl sulfone, a simple probe , was analyzed on alkyl (C IS), phenyl,
phenyl hexyl, and Biphenyl columns to determine th e relative retent ion of each
ph ase, as measu red by capacity factor (k'). In order to ensure separatio n of analytes
from unretained contaminants, a minimum k' value of 2 is recomme nded for most
analyses, however in cases where there is little to no matrix interference, a k' of I
may be acceptable. Th e data in Figur e I show that ph enyl sulfone is retained to a
mu ch great er degree on the Pinnacle?" DB Biphenyl column, than on the other
ph ases tested (k'
=
2.0S). This is due to the un ique retention mechanism of the
biphenyl stationary ph ase, which can interact with both th e hydrophobic aroma tic
ring and the hydrophilic sulfone group through
TC-TC
interaction s. Although the
ph enyl station ary ph ase also allows for the use of
TC-TC
interaction s, the biphenyl
ph ase has a larger electron cloud and is significantly more retentive.
To fur ther test the retenti on of the Biphenyl column, a second set of probes, con
sisting of compounds in the NSAID family, was analyzed. Tenoxicam , which con
tains a sulfone group, and sulfinpyrazone, which contains a sulfoxide group, were
analyzed alon g with a void marker (urac il). Although the se compounds are more
complex than the probe used in the first experiment, the same patt ern of retenti on
was observed (Figur e 2). The Pinnacle" ! DB Biphenyl column exh ibited the great
est retention for tenoxicam. With k' values of 0.33 on the C IS and 0.49 on th e
ph enyl columns, tenoxicam shows almost no retenti on on these stationary pha ses.
The phenyl hexyl ph ase performed slightly bett er with a k' value of 1.52 for tenoxi
cam . However, when tenoxicam was analyzed on th e Biphenyl column under th e
same condition s, the k' value increased to 2.22, a value much mor e likely to pro vide
adequate resolution from mat rix compo nents. Sulfinpyrazone, a less pola r com
pound, also followed th e same pattern of retention (Table I).
The imp roved retention for hydrophilic aromatics shown here is du e to th e unique
TC -TC
interaction retention mechanism of th e Biphenyl ph ase. This mechanism is
particularly useful for analysis of sulfone- and sulfoxide-containing drug com
po unds, which are not easily retain ed on alkyl or ph enyl phases. The Biphenyl
pha se provides greater retention than alkyl and phenyl ph ases and is ideal for sep
arating difficult-to -retain pola r arom atics from unretained matrix contaminants.
Global RESTEK Advantage
· 10·
Figure 1
The Biphenyl phase is more reten
tive for phenyl sulfon e th an oth er alkyl and
phenyl phases.
2.5
k'
_ C18 _ Phenyl _ Phenyl Hexyl _ Biphenyl
Biphenyl columns are much
more effective than alkyl,
phenyl, or phenyl hexyl
phases when increased
retention of hydrophilic
aromatics is desired.
Pinnacle"
1.91Jm
columns
available.
Contact your
local Restek
representative.
DB
www.restek.comWebsite :
www.chromtech.net.auE-mail :
info@chromatech.net.auTelNo : 03 9762 2034 . . . in AUSTRALIA











