Restek Corporation • (800) 356-1688 • (814) 353-1300 •
length, 206nm. The Pinnacle II
™
C8 column performedwell at
284nm, anotherwavelength commonly used for analyzing narcotics.
At 206nm the Pinnacle II
™
C8 column exhibited significant noise
and took excessive time to equilibrate, due to the competingmobile
phase constituents.A longer equilibrationmight have solved the
problem. Both columnsmeet the criteria—with the exception of
thewavelength changewith the Pinnacle II
™
column—forUSP 25
system suitability.
Overall both columns behaved extremelywell in performing the
USP 25 oxycodoneHCl rawmaterialmethod. Howevermany
aspects of themethod appear redundant andmight actually be
compromising the separation. In addition, some of the reagents,
such asTEA,might not be necessary formodern columns. The
fewer reagents amobile phase contains, the smaller the control that
should be needed to affect a robust and practical separation.After
performing theUSP 25method aswritten, wemade some tests to
determine actual needs to achieve the system suitability requirements
as specified.
The first step in simplifying a convoluted analysis is to apply the
KISS principle (Keep it Simple, Scientist!).With peak shape, separa-
tion, and proper analytical technique inmind, we attempted to elimi-
nate some of the perceived problems.We realized that by using 284
nm as the detectionwavelengthwemight not see some impurities,
but in real life thematerial should be tested against some known
source for potency. Note that with the additional reagents removed,
both columns provided good results at the 206nmwavelength.
Next we removed the ion pairing agent and theTEA.We elected to
keep a 20mmolar phosphate buffer system tomaintain a pH of 2.5.
Thenwe reduced the temperature to 27°C. This reduced fluctuations
in retention time caused by changes in air temperature (i.e., air con-
ditioning), and prevented the increase inmass transfer and solubility
in themobile phase frommasking other potential problems. The
temperature change also helped promote column longevity; phos-
phate buffers tend to dissolve silicamore readily at higher tempera-
tures.
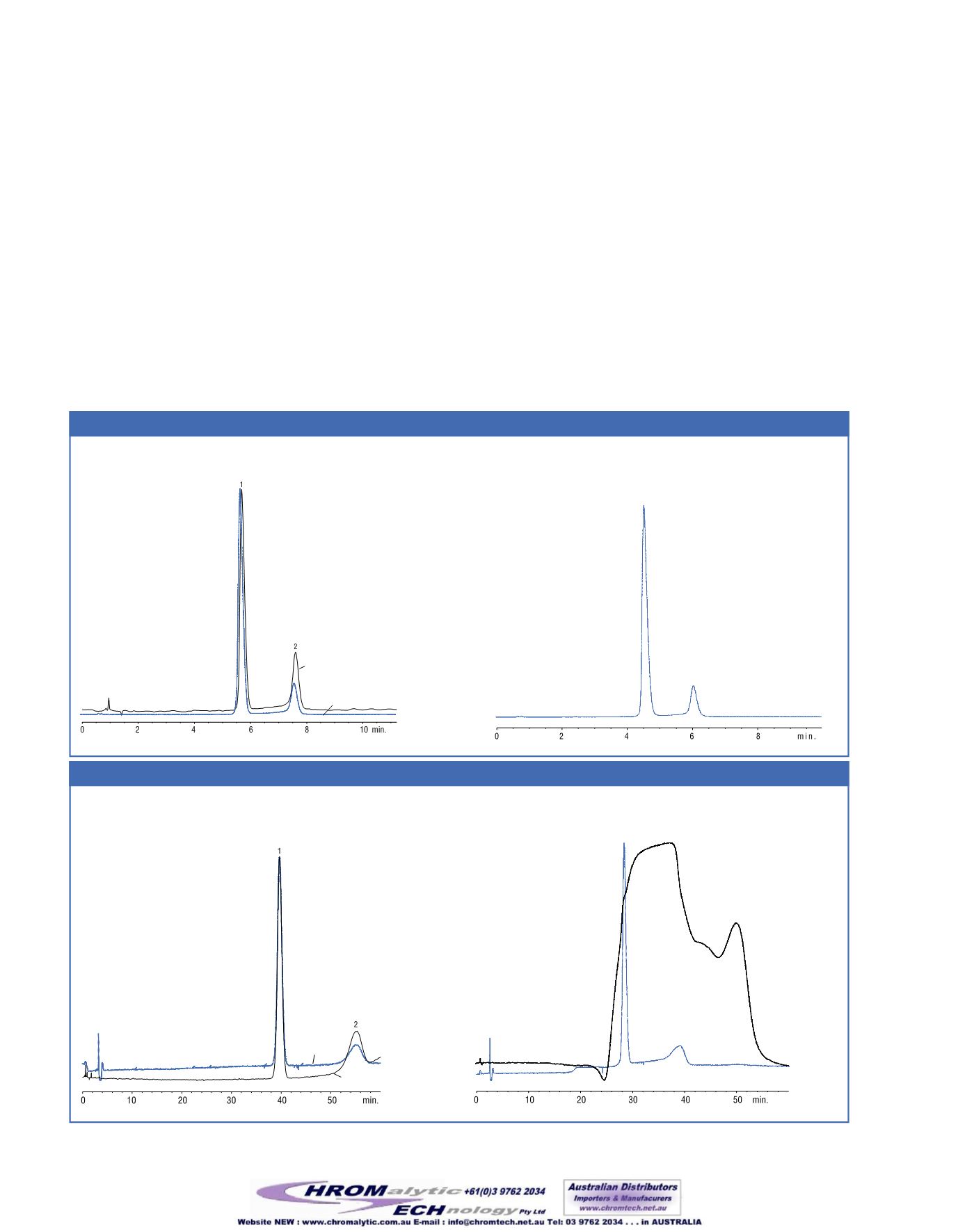
These changes led to a slight increase in tailing for all compounds
on both columns, but the differencewas acceptable, especially
because the run time for the analysiswas reduced by a factor of 3
and resolutionwas improved by 59% to 79%(Application 2). The
system passed the system suitability requirements set forth in the
USPmonograph.
In the next experiment, we re-introduced the ion pair reagent hexane
Ion pair agents andTEA removed—run time decreases 3x and resolution improves.
Application 2
PeakList:
Conc.
(µg/mL)
1. codeine phosphate
13
2. oxycodoneHCl
9
Sample:
Inj.:
10µL
Sample:
resolution solution
Solvent:
mobile phase
Conditions:
Mobile phase: A: 20mM potassium
phosphatemonobasic
pH=2.5
B: methanol
(90A:10B, v/v)
Flow:
1.5mL/min
Temp.:
27°C
Det.:
UV@ 206nm and 284nm
LC_0215
Column: Pinnacle II
™
C8
Catalog #: 9213564
Dimensions: 150 x 4.0mm
Particle size: 5µm
Pore size:
110Å
LC_0210
Column:
UltraC8
Catalog #:
9103564
Dimensions: 150 x 4.0mm
Particle size: 5µm
Pore size:
100Å
Hexane sulfonic acid plus a controlled buffer system doubles analysis time.
Application 3
PeakList:
Conc.
(µg/mL)
1. codeine phosphate 13
2. oxycodoneHCl
9
Sample:
Inj.:
10µL
Sample:
resolution solution
Solvent:
mobile phase
Conditions:
Mobile phase: A: 20mM potassium phosphate
monobasic and 5mm hexane sulfonic acid
inwater pH adjusted to 2.5
B: methanol
(90A:10B, v/v)
Flow:
1.5mL/min
Temp.:
27°C
Det.:
UV@ 206nm and 284nm
LC_0217
Column: UltraC8
Catalog #: 9103564
Dimensions: 150 x 4.0mm
Particle size: 5µm
Pore size:
100Å
LC_0216
Column:
Pinnacle II
™
C8
Catalog #: 9213564
Dimensions:150 x 4.0mm
Particle size:5µm
Pore size: 110Å
206nm
284nm
284nm
206nm
284nm
284nm
206nm
1
2
1
2


