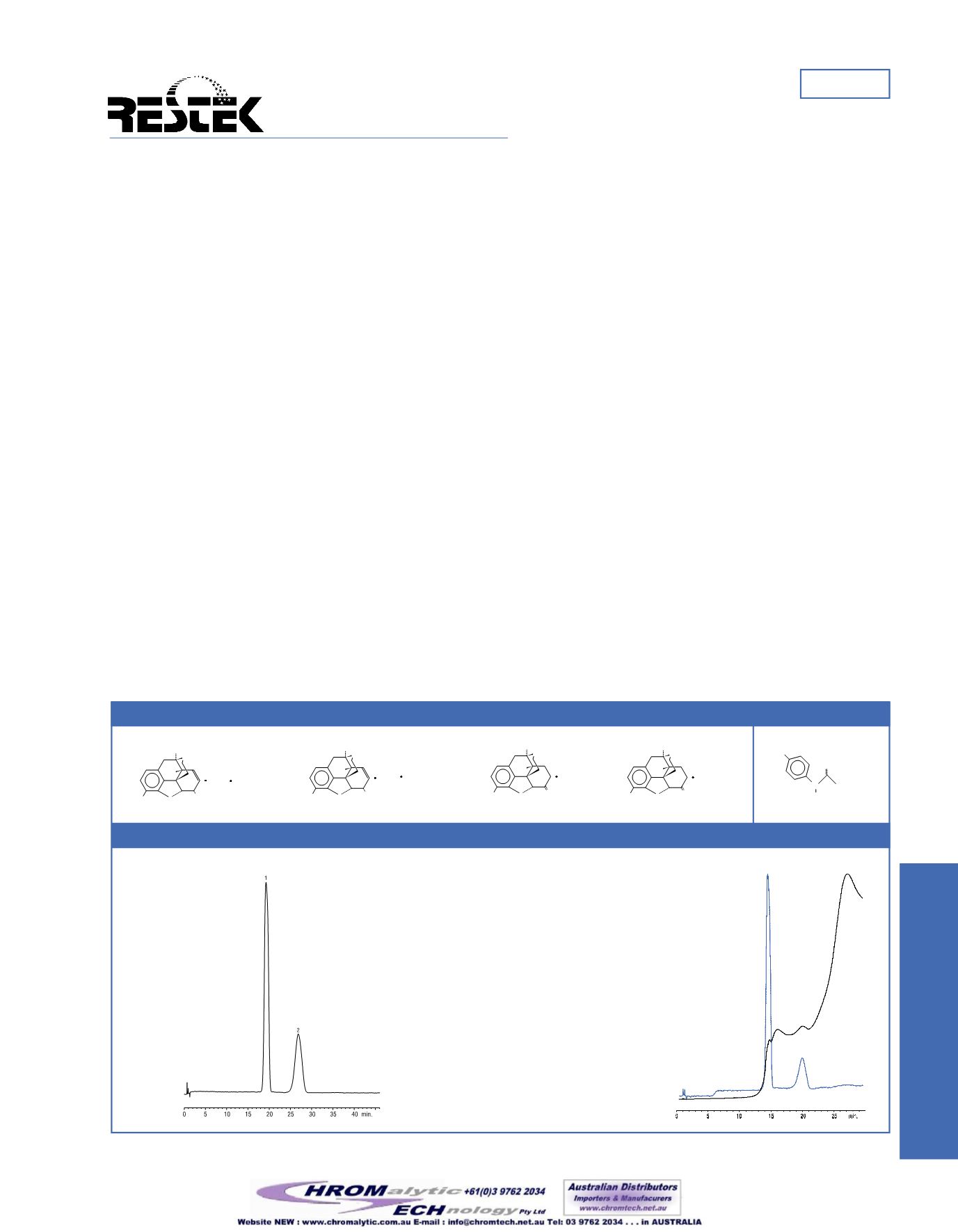
Narcotics andAPAP.
Figure 1
Pharmaceutical
Applications
note
Restek Corporation • (800) 356-1688 • (814) 353-1300 •
#59453
Pharmaceutical
Analysis of Narcotics andNarcotic / AcetaminophenAdmixtures:
What todoWhenCompendiumMethodsDon'tWork
At some point in their careers, especially if performing rawmaterials
or generic testing analyses for pharmaceuticals, analytical chemists
are referred to compendiummethodologies,most often to theUnited
States Pharmacopoeia (USP), theEuropeanPharmacopoeia (EP), or
theBritishPharmacopoeia (BP), but occasionally to other volumes.
Often themethods described in these compendia provide less than the
desired robustness in separation and reproducibility, or the results
may bemarginal—barely passing system suitability requirements.
Sometimes a particular delivery system formulation absolutelywill
not workwith a genericmethod, due to interference from other ingre-
dients in the sample.Modificationsmust bemade to the problem
methodology, and the results compared statistically to the original. To
improve analysis efficiency and reduce laboratory supply costs asso-
ciatedwith revalidating and testing amethod, itmay be desirable to
create and validate a single analyticalmethod for awide range of
similar drug products.
Many narcotics are very similar in structure, often varying by only a
single substitution.Morphine, codeine, hydrocodone, and oxycodone
are quite similar, for example (Figure 1). Some of these closely relat-
ed compounds—all butmorphine, in fact—might be blendedwith
other analgesics, such as acetaminophen (APAP). However, USP 25
describesmore than 7 differentmethods to test these rawmaterials
and admixtures, and some of these older rawmaterialmethods do not
useHPLC as a primary test for purity.
One of the chromatographic applications inUSP 25 is for the analy-
sis of oxycodone rawmaterial.After reading themobile phase sec-
tion, which lists sulfonic acids, triethylamine, water, phosphoric acid
andmethanol as the constituents, we saw potential problemswith the
method, including:
1) The use ofmethanol in this analysis can lead to high background
absorption and loss of linear range, because the analytical wavelength
is 206nm, and theUV cutoff point formethanol is 235nm. In extreme
cases this also can reduce sensitivity, because the lamp is a finite
energy source—themore energy the background absorbs, the less is
available to the sample.
2) An ion-pairing agent (heptane sulfonic acid) is introduced into the
mobile phasewithout a proper buffer tomaintain pH at a known
level. This situation can lead towidened peaks, tailing peaks, and
retention time drift. The goal of ion pairing is to create a “neutral”
species.
3) TEAmodifier is included in themethod.When basic compounds
are analyzed on older-typeHPLC columns, TEA often is added as
competing base, to reduce the tailing caused by acidic silanol activity.
If the analytical species are neutral, or have been “neutralized” by the
addition of an ion-pairing agent, the addition of TEA should have no
beneficial effect. SinceTEA is a base, adding it to amobile phase
containing sulfonic acidswill instantly cause an acid/base neutraliza-
tion, producing a salt andwater and reducing the effective concentra-
tion of the acidic ion-pairing agent. This reaction could lead to the
formation of undesirable side products in themobile phase that also
will absorb in the lowUV range, creating noisy baselines.
Furthermore, TEA is volatile, and its compositionmight change over
time if themobile phase is sparged.
With these concerns inmind, we tested theUSPmethod, using our
Ultra andPinnacle II
™
C-8 columns (Application 1).
Initial analyseswere performed according toUSP 25. TheUltraC8
column gave the better performance at the specified detectionwave-
OxycodoneHCl rawmaterial monograph performed perUSP 25.
Application 1
HO
N
H
CH
3
O
Acetaminophen
O
CH
3
O
OH
NCH
3
H
H
CodeinePhosphate
H
3
PO
4
H
2
O
O
CH
3
O
NCH
3
H
O
Bitartrate
H
HydrocodoneBitartrate
O OH
NCH
3
H
H
HO
H
2
SO
4
H
2
O
MorphineSulfate
O
CH
3
O
NCH
3
H
O
HCl
HO
OxycodoneHCl
LC_0213
PeakList:
Conc.
(µg/mL)
1. codeine phosphate
13
2. oxycodoneHCl
9
Sample:
Inj.:
10µL
Sample:
resolution solution
Solvent:
mobile phase
Conditions:
Mobile phase: A: 5mM hexane sulfonic acid
with 2mLTEA and 3mL of
phosphoric acid per liter.
pH adjusted to 2.5
B: methanol
(90A:10B, v/v)
Flow:
1.5mL/min
Temp.:
40°C
Det.:
UV@ 206 and 284nm
LC_0214
Column: Pinnacle II
™
C8
Catalog #: 9213564
Dimensions: 150 x 4.0mm
Particle size: 5µm
Pore size:
110Å
Column: UltraC8
Catalog #: 9103564
Dimensions:150 x 4.0mm
Particle size:5µm
Pore size: 100Å
284nm
206nm
1
2
206nm


