HPLC
Applications
note
RestekCorporation • (800)356-1688 • (814) 353-1300 •
#59177
HPLC
AnalyzePolar CompoundsbyReversedPhaseHPLCUsingUltraAqueous
C18Column
Short-chainorganic acids, amino acids, andwater-soluble
vitamins are examples of highlypolar compounds that are
difficult to retainusingconventional reversedphasecolumns,
evenwith littleor noorganic solvent in themobilephase. The
UltraAqueousC18 columnwas designed for reversedphase
applications that requirehighly aqueousmobilephases.The
analysis of sixorganic acids,which aredifficult to retainusing
manyconventionalC18columns, shows theUltraAqueousC18
columnprovides enhanced retention and selectivity (Figure1).
TheUltraAqueousC18 column alsogives reproducible retention
times, sharper peak shapes; allows useof highly aqueousmobile
phases, andmay eliminate theneed for samplederivatizationor
ionpairing reagents.
ReproducibleRetentionTimes
Whenexposed tohighlyaqueousmobilephases,manyconven-
tionalC18 columnswill lose retention from run to runbecause of
aphenomenon called “chain folding.”This loss of retention can
begradual or sudden and is attributed to thehydrophobicC18
chains self-associating (i.e., foldingdownonto the silica surface
to avoid associatingwith averyhydrophilicmobilephase).
While the chain foldingprocess is reversible, it canmake
analyses of highlypolar compounds difficult due to
irreproducible retention times.
TheUltraAqueousC18column, however, avoids thisproblem—
it provides stable and reproducible retention, evenwith100%
aqueousmobilephases. TheUltraAqueousC18 column is
designedusingTypeB, high-purity silica and anovel bonding
chemistry that results in a trueC18phase (USPL1). Because
polar groups on the silica surface keep the stationaryphasewet,
the alkyl chains remain totally extended evenwhen continually
exposed to ahighly aqueousmobilephase. This unique second-
arypolar character prevents chain folding, and enhances the
retention and selectivityof polar compoundswithout compro-
mising the level of basedeactivation.
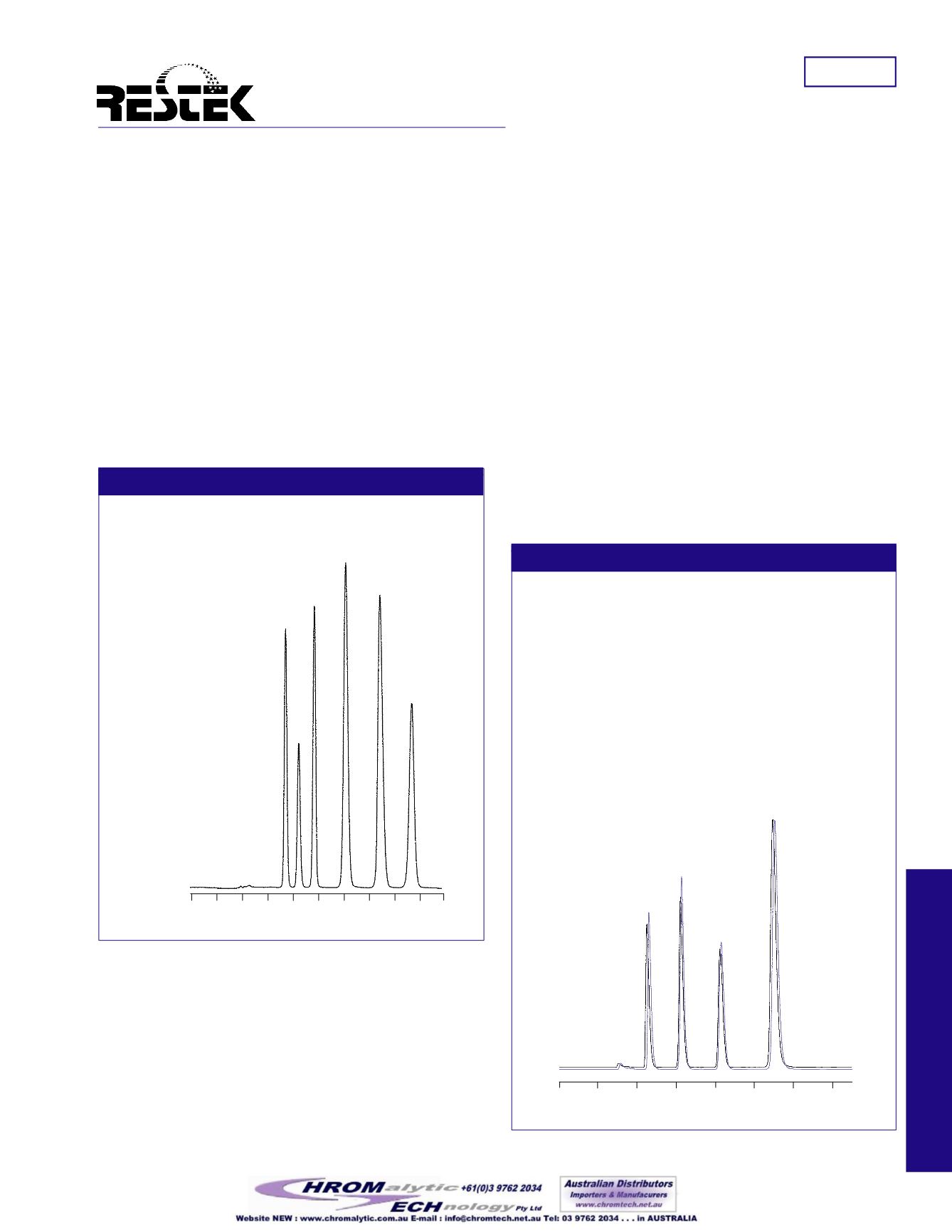
Notice thehighly reproducible separationof four small carboxy-
lic acids using theUltraAqueousC18 column and a 100%
aqueousmobilephase (Figure2).No significant change in
retentionoccurredover twenty injections, performedover a four-
dayperiod that included threedays of continuous exposureof the
column to the totally aqueousmobilephase.
PeakList
Conc.
(mg/mL)
1. glycolic acid
5.4
2. malonic acid
4.2
3. acetic acid
7.8
4. maleic acid
0.06
Sample dissolved inmobile phase.
Column:
UltraAqueousC18Column
Catalog#:
9178565
Dimensions:
150x4.6mm
ParticleSize:
5µm
PoreSize:
100Å
Conditions:
MobilePhase:
50mMpotassium
phosphate, pH 2.5
FlowRate:
1.0mL/min.
Temp.:
25°C
Det.:
UV@210nm
Inj.:
10µL
1
2
3
4
0
1
2
3
4
5
6
7min.
LC_0142
Figure2
UltraAqueousC18 columnachieves stable retentionof
organic acids in 100% aqueousmobile phase.
Figure1
UltraAqueousC18 column provides enhanced retention
and selectivity for organic acids.
PeakList:
Conc.
(µg/mL)
:
1. malonic acid
500
2. lactic acid
500
3. acetic acid
1000
4. citric acid
1000
5. succinic acid
2000
6. fumaric acid
10
Sample:
Solvent:
HPLC- gradewater
Inj.:
10µL
Column:
UltraAqueousC18
Catalog#:
9178565
Dimensions: 150 x 4.6mm
Particle size: 5µm
Pore size:
100Å
Conditions:
Mobile phase: 50mMpotassium
phosphate, pH 2.5:
acetonitrile (99:1)
Flow:
1.5mL/min.
Temp.:
25°C
Det.:
UV@210nm
LC_0140
1
2
3
4
5
6
0
1
2
3
4
5 min.
A. initial
B. after 3Days


