Restek Corporation • (800) 356-1688 • (814) 353-1300 •
Figure 4—Use theNLEAFAMEMix to standardize
Fat-by-FattyAcidCompositionmethods, such asAOAC 996.06.
analysis of amarine-oil FAME standard; amarine oil sample is
shown in Figure 2. Both analyses are characterized by fast, effec-
tive resolution and sharp, symmetric peaks.
Resolving
cis
and
trans
Isomers
Individual
cis
and
trans
isomers are resolved on a 100-meter Rt-2560
column,making this the column of choice for analyzing partially
hydrogenated fats. The highly polar biscyanopropyl phase gives the
selectivity needed for resolvingFAMEs isomers, such as the
cis
and
trans
forms of C18:1. The
trans
isomers elute before the
cis
isomers
on this phase, opposite of the elution order onCarbowax
®
-based
phases such as FAMEWAX
™
or Rtx
®
-Wax. Figure 3 shows the chro-
matographic separation of 37FAMEs typically encountered in veg-
etable, animal, andmarine fats and oils, using anRt-2560 column.
AOACmethod 996.06
1
specifies the determination of total fat content
based on the fatty acid content, after conversion to themethyl esters.
This is the specifiedmethod for determining total fat content for
nutritional labeling purposes.After quantifying the total FAMEs pre-
sent in the derivatized sample, the amount of fat (as triglycerides) in
the sample is calculated, based on initial sampleweight. The 100-
meter Rt-2560 columnmeets the requirements of this procedure.
This column also allows quantification of the total
trans
content.
To calibrate theGC system for assays of this type, use a FAMEmix-
ture such as our 37-component Food IndustryFAMEMix (Figure 3)
or our 28-component NLEAFAMEMix (Figure 4). Both standards
include a gravimetric certificate of analysis to help ensure accurate
quantification. To ensure correct identifications of the individual
cis
and
trans
isomers of C18:1, use our
cis/trans
IsomerMix, as shown
inFigure 5.
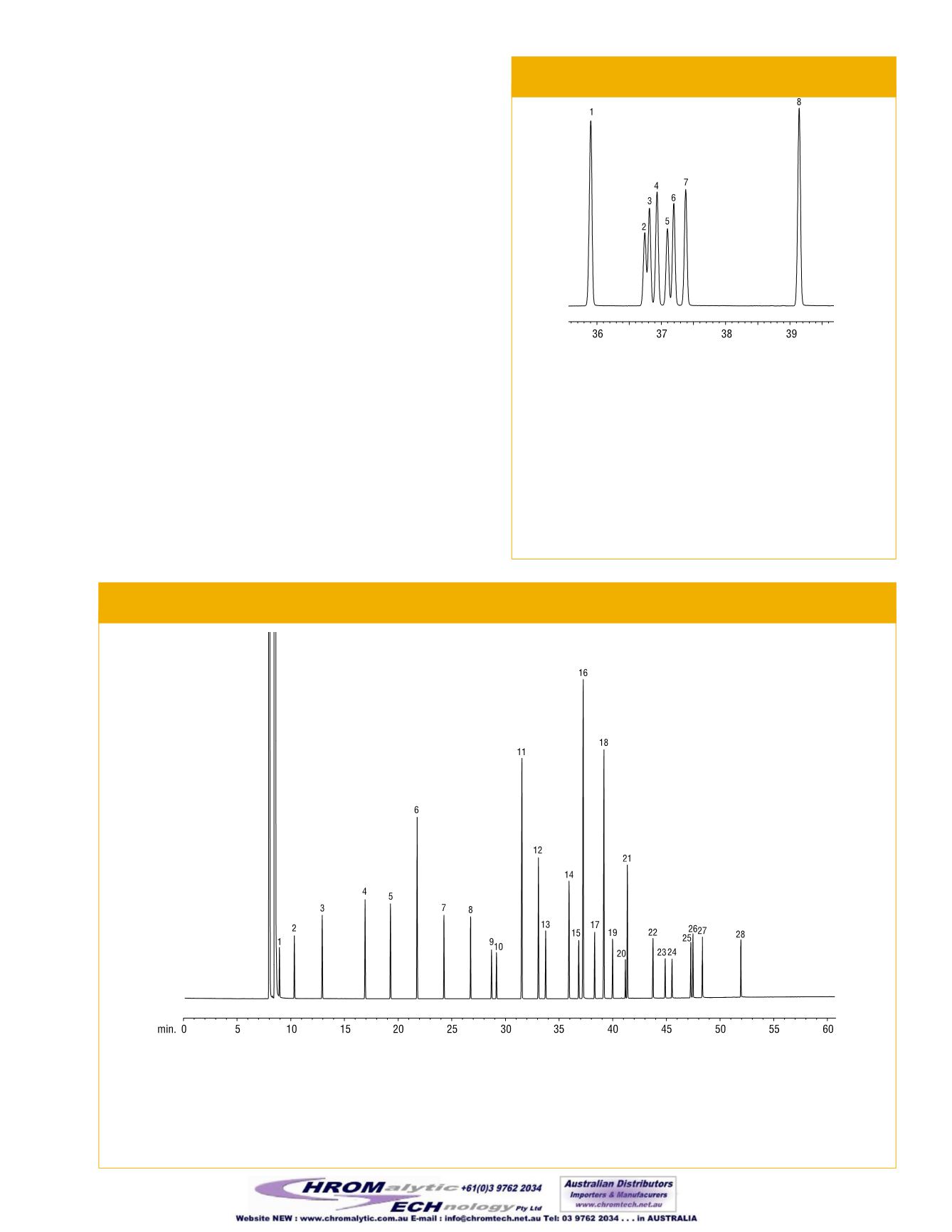
Figure 5—Resolve
cis
and
trans
isomers of
unsaturated FAMEs on anRt-2560 column.
GC_FF00652
Rt-2560, 100m, 0.25mm ID, 0.2µm (cat.# 13199)
Sample:
10mg/mL total FAMEs inmethylene chloride
Inj.:
1.0µL split (split ratio 20:1), 4mm inlet liner (cat.# 20814)
Inj. temp.:
225°C
Carrier gas:
hydrogen, constant flow
Flow rate:
1.2mL/min.
Oven temp.:
100°C (4min. hold) to 240°C@ 3°C/min. (10min. hold)
Det.:
FID@ 250°C
Compound
% inMix
1. C18:0 methyl stearate
20.0
2. C18:1methyl petroselaidate (
trans
-6)
8.0
3. C18:1methyl elaidate (
trans
-9)
10.0
4. C18:1methyl transvaccenate (
trans
-11) 12.0
5. C18:1methyl petroselinate (
cis
-6)
8.0
6. C18:1methyl oleate (
cis
-9)
10.0
7. C18:1methyl vaccenate (
cis
-11)
12.0
8. C18:2methyl linoleate (
cis
-9,12)
20.0
Column:
Rt-2560 100m, 0.25mm ID, 0.20µm
(cat.# 13199)
Sample:
30mg/mL total FAMEs inmethylene chloride
Inj.:
1.0µL split (split ratio 100:1),
4mm inlet liner (cat.# 20814)
Inj. temp.:
225°C
Carrier gas:
hydrogen, constant flow
Flow rate:
1.2mL/min.
Oven temp.:
100°C (4min. hold) to 240°C
@3°C/min. (10min. hold)
Det.:
FID@250°C
1. C4:0 methyl butyrate
2. C6:0 methyl heanoate
3. C8:0 methyl octanoate
4. C10:0 methyl decanoate
5. C11:0 methyl undecanoate
6. C12:0 methyl laurate
7. C13:0 methyl tridecanoate
8. C14:0 methyl myristate
9. C14:1 methyl myristoleate (
cis
-9)
10. C15:0 methyl pentadecanoate
11. C16:0 methyl palmitate
12. C16:1 methyl palmitoleate (
cis
-9)
13. C17:0 methyl heptadecanoate
14. C18:0 methyl stearate
15. C18:1 methyl elaidate (
trans
-9)
16. C18:1 methyl oleate (
cis
-9)
17. C18:2 methyl linoelaidate (
trans
-9,12)
18. C18:2 methyl linoleate (
cis
-9,12)
19. C20:0 methyl arachidate
20. C20:1 methyl eicosenoate (
cis
-11)
21. C18:3 methyl linolenate (
cis
-9,12,15)
22. C22:0 methyl behenate
23. C22:1 methyl erucate (
cis
-13)
24. C23:0 methyl tricosanoate
25. C24:0 methyl lignocerate
26. C20:5 methyl eicosapentaenoate
(
cis
-5,8,11,14,17)
27. C24:1 methyl mervonate (
cis
-15)
28. C22:6 methyl docosahexaenoate
(
cis
-4,7,10,13,16,19)
GC_FF00651


