
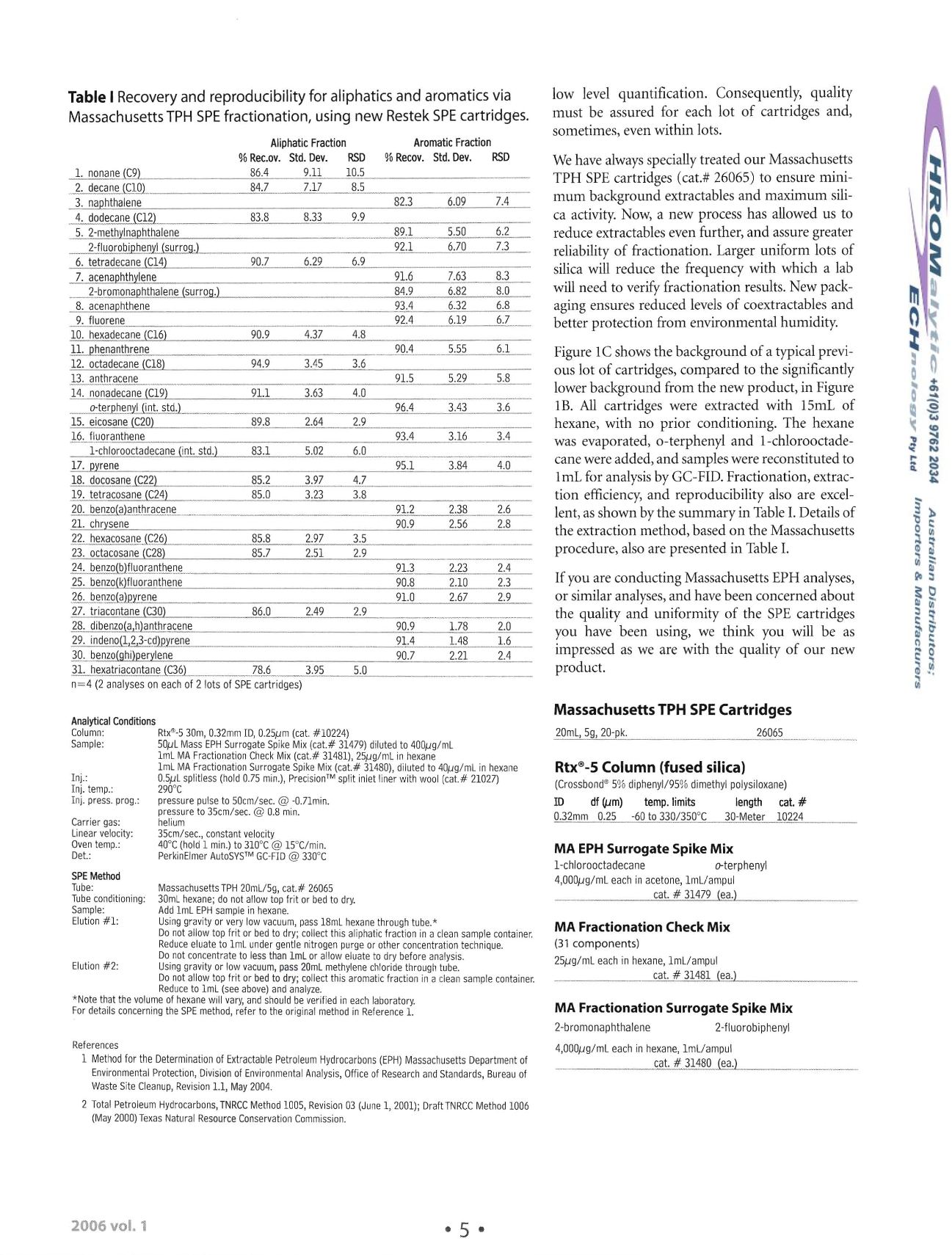
Table I
Recovery and reproducibility for aliphaticsand aromatics via
low level qu ant ification . Consequently, quality
must be assured for each lot of cartridges and,
Massachusetts TPH SPE fractionation, using new Restek SPE cartridges.
sometimes, even with in lots.
AliphaticFraction
Aromatic Fraction
%Rec.ov, Std. Dey.
RSD
%
Recov,
Std.Dey.
RSD
We have always specially treated our Massachusetts
1. nonane (C9)
86.4
9.11
10.5
TPH SPE cartrid ges (cat.# 26065) to ensure mini. 2. decane
(t:.1QL
84.7
7.17
8.5
mum backgrou nd extra ctables and maximum sili
3. naphthalene
82.3
6.09
7.4
4. dodecane C12
83.8
8.33
9.9
ca activity. Now, a new process has allowed us to
5. 2-methylnaphthalene
89.1
5.50
6.2
reduce extractables even furth er, and assure greater
2-fluorobiphenyl (surrog.}
92.1
6.70
7.3
reliability of fractionation. Larger uniform lots of
6. tetradecane (C14)
90.7
6.29
6.9
silica will redu ce the frequency with which a lab
7. acenaphthylene
91.6
7.63
8.3
2-bromonaphthalene (surro .
84.9
6.82
8.0
will need to verify fractionation results. New pack
8. acenaphthene
93.4
6.32
6.8
aging ensures reduced levels of coextractables and
9. fluorene
92.4
6.19
6.7
better protection from environmental humidity.
10. hexadecane (Cl6)
90.9
4.37
4.8
11. phenanthrene
90.4
5.55
6.1
Figure IC shows the background of a typical previ
12. octadecane (C18)
94.9
3.45
3.6
ous lot of cartridges, compared to the significantly
l L .anthracene
91.5
5.29
5.8
lower background from the new product, in Figure
14. nonadecane (Cl9)
91.1
3.63
4.0
o-terphenyl int. std.
96.4
3.43
3.6
l B. All cartridges were extracted with 15mL of
15. eicosane C20
89.8
2.64
2.9
hexane, with no prior conditioning. The hexane
1&:
fluoranthene
93.4
3.16
3.4
was evapora ted, o-terphenyl and l -chlorooctade
1-chlorooctadecane (int. std.)
83.1
5.02
6.0
-~~-~----
cane were added, and sampleswere reconstitut ed to
1L.Pyrene
95.1
3.84
4.0
18. docosane C22
85.2
3.97
4.7
ImL for analysis by GC-FID. Fraction ation, extrac
19. tetracosane (C24)
85.0
3.23
3.8
tion efficiency, and reproducibility also are excel
20.
benzo(a~'!!hLasene
91.2
2.38
2.6
lent, as shown by the summary in Table
I.
Details of
21. chrysene
90.9
2.56
2.8
the extraction method, based on the Massachusetts
12. hexacosane
(<:.~)
85.8
2.97
3.5
procedure, also are presented in Table
I.
23. octacosane (C28)
85.7
2.51
2.9
24. benzo(b)fluorantheQ.?
91.3
2.23
2.4
If you are cond ucting Massachusetts EPH analyses,
l.~jJ~lgQl~)pyrene
91.0
2.67
2.9
or similar analyses, and have been concerned about
25. benzo(k)fluoranthene
90.8
2.10
2.3
27. triacontane 0 0
86.0
2.49
2.9
the quality and uniformity of the SPE cartridges
._-~-~-
28. dibenzo(a,h)anthracene
90.9
1.78
2.0
you have been using, we think you will be as
29. indeno(l, 2,3-cd)pyrene
91.4
1.48
1.6
impressed as we are with the quality of our new
30. benzo(ghi)perylene
90.7
2.21
2.4
31. hexatriacontaneJ C36)___
~
_ _J8 .6
3.95
5.0
product.
n=4 (2 analyses on eachof 2 lots of SPEcartridges)
Massachusetts TPH SPE Cartridges
Analytical Conditions
Column:
Rtx"-S30m, 0.32mm!D, 0.2S/lm(cat. # 10224)
20mL, 5g, 20-pk.
26065
Sample:
SO/lL Mass EPH SurrogateSpike Mix (cat.# 31479) dilutedto
400/lg/mL
l mLMAFractionationCheckMix (cat.# 31481), 2S/lg/mLin hexane
l mLMAFractionationSurrogate Spike Mix(cat.# 31480), dilutedto
40/lg/mL
in hexane
Rtx<!l-S Column(fusedsilica)
Inj.:
O.S/lL splitless (holdO.7Smin.), Precision™split inlet liner withwool (cat.# 21027)
(Crossbond"
5%diphenyl/ 95%dimethyl polysiloxane)
Inj.temp.:
290°C
Inj. press. prog.: pressurepulseto
SOcm/sec.
@
-0.71min.
ID
df (,um)
temp. limits
length
cat.
#
pressureto35cm/sec.
@
0.8 min.
0.32mm 0.25 -60 to 330/350°C
10,,,,,-
3D-Meter
,"" 224,--~
__
Carrier gas:
helium
Linear velocity:
3Scm/ sec., constant velocity
Oventemp.:
40°C(hold1 min.) to310°C
@
lSoCimin.
MA EPH Surrogate Spike Mix
Del.:
PerkinElmer AutoSYSTMGC'F!D
@
330°C
1-chlorooctadecane
o-terphenyl
SPEMethod
4,OOO/lg/ mL
each in acetone, 1mL/ ampul
Tube:
MassachusettsTPH20mL/Sg, cat.# 2606S
cat. # 31479
(ea.)
Tube conditioning: 30mLhexane; donot allow topfrit or bed to dry.
Sample:
Addl mLEPH samplein hexane.
Elution# 1:
Using gravity or verylowvacuum, pass18mLhexane throughtube*
MA Fractionation Check Mix
Do not allowtopfrit or bed to dry; collectthisaliphaticfractionina cleansample container.
Reduce eluate tol mLunder gentle nitrogen purge orother concentration technique.
(31 components)
Do not concentrate to lessthanl mLor allow eluate to dry before analysis.
25/l g/ mLeach in hexane, 1mL/ampul
Elution #2:
Usinggravity or low vacuum, pass20mLmethylene chloride throughtube.
Do not allowtopfrit or bedto dry; colectthis aromatic fractionina clean sample container.
cat. # 31481 (ea.)
Reducetol mL(seeabove) andanalyze.
*Note that the volume ofhexanewill vary, and shouldbeverifiedineach laboratory.
For detailsconcerningtheSPEmethod, refer to the original methodin Reference
1.
MA Fractionation Surrogate Spike Mix
2-bromonaphthalene
2-fluorobiphenyl
References
4,OOO/lg/ mLeach in hexane, 1mL/ ampul
1 Methodfor theDetermination of ExtractablePetroleumHydrocarbons(EPH) Massachusetts Department of
cat.
#
31480 (ea.)
Environmental Protection, Divisionof Environmental Analysis, Ofice of Research andStandards, Bureau of
WasteSiteCleanup,Revision
1.1,
May 2004.
2 Total PetroleumHydrocarbons,TNRCCMethod100S, Revision 03(June1, 2001); Draft TNRCCMethod1006
(May 2000)Texas NaturalResourceConservationCommission.
2006
vol. 1
• 5 •Website :
www.chromtech.net.auE-mail :
info@chromatech.net.auTelNo : 03 9762 2034 . . . in AUSTRALIA











