
•
8
•
2007 vol. 4
Clinical/Forensics
Simplify and Speed Up Opiates Analysis
Using LC/MS/MS & an Allure® PFP Propyl HPLC Column
By Kristi Sellers, Innovations Chemist
• 7-minute analysis time, for increased
sample throughput.
• Faster sample prep—no derivatization
required.
• Separate compounds with similar mass
spectra.
Opiates are one of the primary drug classes tested
in clinical and forensic laboratories, and most con-
firmation methods use GC/MS. These methods
require derivatization of the target compounds,
which significantly lengthens sample preparation
time. Here we present an alternative confirmation
method, using LC/MS/MS, which can increase
sample throughput by eliminating derivatization
and shortening analysis time. This procedure also
provides accurate confirmation and quantification
of compounds that have similar mass spectra, by
using an Allure® PFP Propyl column to chromato-
graphically separate compounds that share product
ions, allowing positive identification based on
retention time.
In developing this LC/MS/MS method for the
analysis of opiates, our goals were to obtain baseline
resolution of compounds having similar mass
spectra while providing an analysis time of less
than 10 minutes. To accomplish this, mass spec-
trometer conditions, column selection, mobile
phase, and gradient profiling were evaluated and
optimized. Several different stationary phases ini-
tially were evaluated including an aqueous C18, a
biphenyl, a propyl cyano, and a pentaflurophenyl
propyl stationary phase. Consistent column dimen-
sions and base silica (50mm, 2.1mm ID, 5µm par-
ticle size, and 60Å pore size) were used for all phases;
mobile phase conditions were optimized for each
stationary phase. Mobile phases tested included:
0.1% formic acid in water, 0.1% formic acid in
acetonitrile, and 0.1% formic acid in methanol in
various combinations. A variety of gradient profiles
also were evaluated.
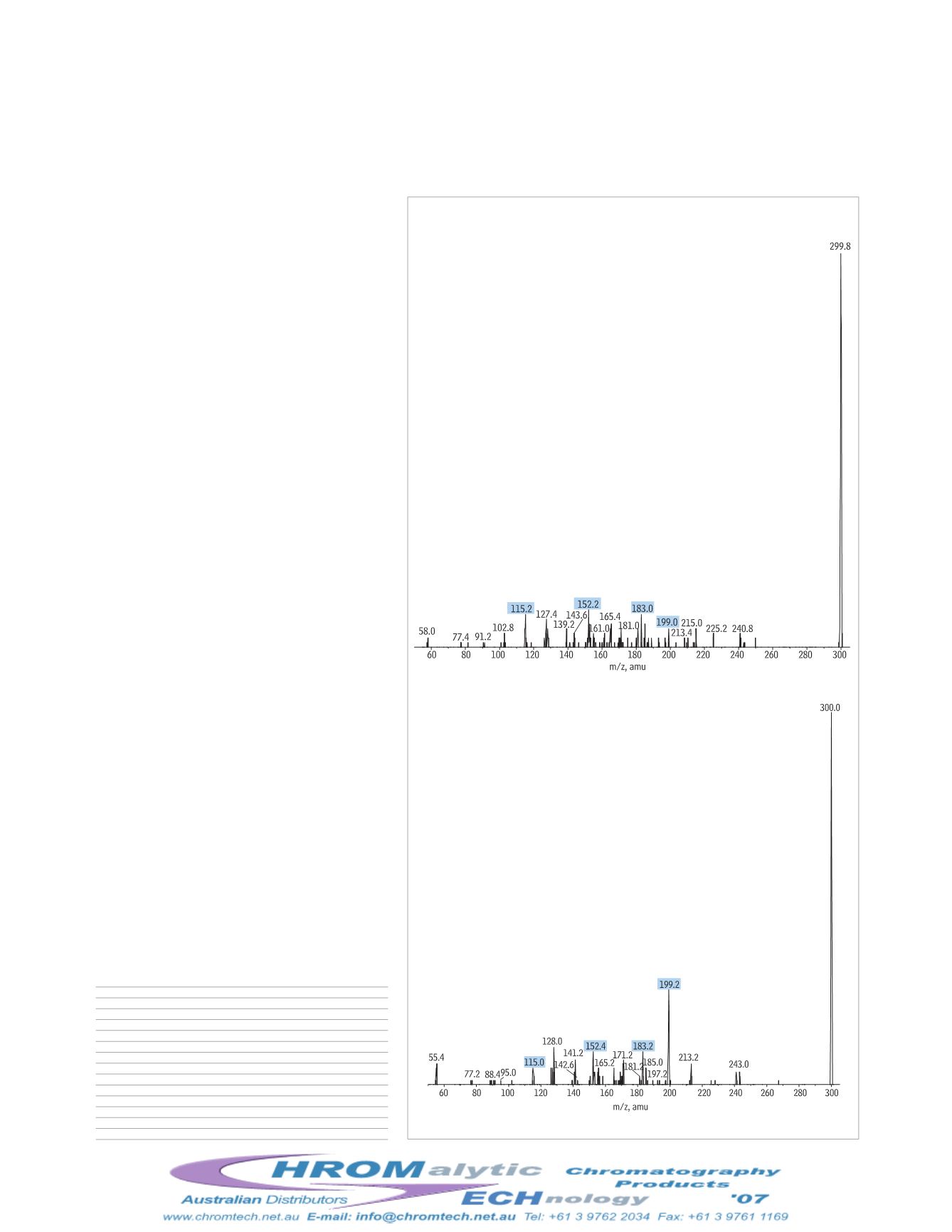
Figure 1
Codeine and hydrocodone share product ions and must
be separated chromatographically.
Table I
+MRM Transitions for Opiates.
LC_PH0457
A. Codeine
LC_PH0458
B. Hydrocodone
Mass Spectrometer Experiments:
Declustering Collision
Compound
Q1
Q3 Potential (V)
Energy (V)
morphine
286 152
46
79
morphine
286 165
46
51
hydromorphone
286 185
46
41
hydromorphone
286 157
46
55
oxymorphone
302 227
36
37
oxymorphone
302 198
36
55
codeine
300 152
46
85
codeine
300 115
46
89
hydrocodone
300 199
46
39
hydrocodone
300 128
46
69
oxycodone
316 240
31
39
oxycodone
316 256
31
33
6-monoacetylmorphine 328 211
51
55
6-monoacetylmorphine 328 193
51
35
For conditions see Figure 2.











