
uccessfully Implement the
Contents
Revised USP
<467>
Method
Continued from page 1.
Pharmaceutical
"'"
V
SuccessfullyImplement the Revised USP
<467> Method (Residual Solvents)
1
III.
Gas Chromatography Accessories
Jill
ProtectYour Dataand Analytical Column
Usinga Restek ElectronicLeakDetector . .. 7
Foods, Flavors
&
Fragrances
PrepareSamples
in Half the Time Using a Fraction
of the Solvent with dSPE
8
"'"
V
Pharmaceutical
Beyond C
18-1
ncreaseRetention
of Hydrophilic Compounds Using
Biphenyl Columns
10
Patents
&
Trademarks
Restek patents andtrademarks aretheproperty of Restek
Corporation. Other trademarksappearing inRestekliteratureor
onitswebsite aretheproperty oftheir respectiveowners.
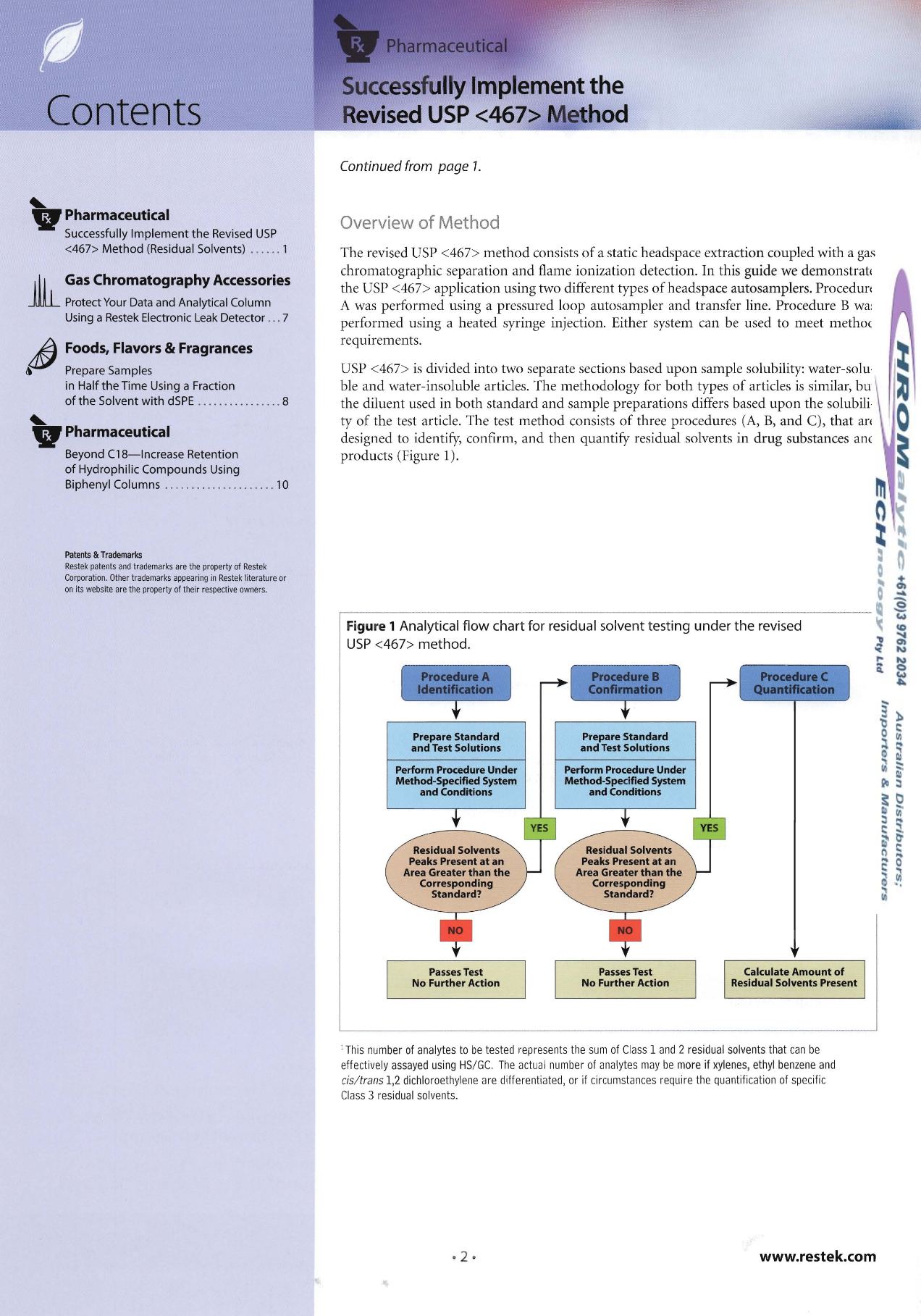
Overview of Method
The revised USP <467> method consists of a static headspace extraction coupled with a gas
chromatographic separation and flame ionization detection . In this guide we demonstrate
the USP <467> application using two different types of headspace autosamplers. Procedur e
A was perform ed using a pressured loop autosampler and transfer line. Procedure B was
performed using a heated syringe injection. Either system can be used to meet method
requirements.
USP <467> is divided into two separate sections based upon sample solubility: water-solu
ble and water-insoluble articles. The methodology for both types of articles is similar, but
the diluent used in both standard and sample preparati ons differs based upon the solubili
ty of the test article. The test meth od consists of three procedures (A, B, and
e),
that are
designed to identify, confirm, and then quantify residual solvents in drug substances and
products (Figure I).
Figu re 1
Analytical flow chart for residual solvent testing under the revised
USP
<467> method.
ProcedureC
Quantification
Prepare Standard
Prepare Standard
and Test Solutions
and Test Solutions
Perform Procedure Under
Perform Procedure Under
Method-Specified System
Method-Specified System
and Conditions
and Conditions
Standard?
Standard?
Residual Solvents
Peaks Present at an
Area Greater than the
Residual Solvents
Corresponding
Peaks Present at an
Area Greater than the
Corresponding
Calculate Amount of
Residual Solvents Present
1
This number of analytes to betested represents the sumof Class
1
and 2 residual solvents that can be
effectively assayed using HS/ GC. The actual numberof analytes may bemore if xylenes, ethyl benzene and
cis/ trans
1,2
dichloroethylene are differentiated, or if circumstances require the quantificationof specific
Class 3 residual solvents.
·2·
www.restek.comWebsite :
www.chromtech.net.auE-mail :
info@chromatech.net.auTelNo : 03 9762 2034 . . . in AUSTRALIA











