
• 12 •
www.restekcorp.com800-356-1688
by Rebecca Wittrig, Ph.D., Food, Flavor & Fragrance Innovations Chemist
High-Resolution Analysis of Fatty
Acid Methyl Esters (FAMEs)
Using an Rt-2560 Capillary GC Column to
Resolve
cis
and
trans
Isomers
✔
Highly polar Rt-2560 column meets analysis requirements of AOAC Method 996.06.
✔
Column suitable for determining fatty acid composition or total
trans
fat.
✔
Reference materials formulated for calibrating the GC system and identifying isomers.
Modern requirements for characterizing fats and
oils and determining the total fat content in foods
call for highly efficient separations offered by capil-
lary GC columns. A properly chosen column can
provide accurate information about complex fat or
oil samples, e.g., total fat content,
trans
fat content,
or total omega-3 polyunsaturated fatty acid content.
Carbowax
®
-type (polyethylene glycol) stationary
phases typically are used for separating, identifying,
and quantifying saturated and unsaturated fatty acid
methyl esters (FAMEs). More polar biscyanopropyl
phases, typically in longer columns, are needed to
resolve
cis
and
trans
isomers of polyunsaturated
components, for determining total fat content, or
for quantifying total
trans
fat.
Individual
cis
and
trans
FAME isomers are effective-
ly resolved on a 100-meter Rt-2560 column, making
this the column of choice for analyzing partially
hydrogenated oils. The highly polar biscyanopropyl
phase gives the selectivity needed for resolving these
isomers, such as the
cis
and
trans
forms of C18:1.
The
trans
isomers elute before the
cis
isomers,
opposite of their elution order on Carbowax
®
-based
phases such as Rtx
®
-Wax or FAMEWAX
®
.
AOAC Method 996.06, the specified method for
determining the total fat content of a food for nutri-
tional labeling purposes, calls for determining total
fat content based on fatty acid content, after the
fatty acids are converted to methyl esters.
1
The 100-
meter Rt-2560 column meets the requirements of
this procedure. An Rt-2560 column also allows
quantification of total
trans
content. Note that Rtx
®
-
2330 columns, our slightly less polar 90% bis-
cyanopropyl phase columns, also resolve
cis
and
trans
FAME isomers. On Rtx
®
-2330 columns, as on
Rt-2560 columns, the
trans
forms of the FAMEs
elute before the
cis
forms.
To calibrate the GC system for assays of this type,
use a FAME mixture such as our Food Industry
FAME Mix, a 37-component mixture of FAMEs typi-
cally encountered in vegetable, animal, and marine
fats and oils, or our 28-component NLEA FAME Mix
(Figure 1). Each of these standards includes a gravi-
metric certificate of analysis to help ensure accurate
quantification. To ensure correct identifications of
individual
cis
and
trans
isomers of C18:1, use our
cis/trans
Isomer Mix.
Whatever your fat or oil analysis requirements,
Restek can provide high-performance analytical
columns and reference standards that will help you
to accurately characterize your materials.
1. Official Methods of Analysis,
17th edition, AOAC International, 2000.
Ordering Information
| Rt-2560 Column (Fused Silica)
ID
df (µm)
temp. limits
100-Meter
0.25mm
0.20
20 to 250°C
13199
Compound
Qty. (%)
C4:0
1.5%
C6:0
1.5%
C8:0
2.0%
C10:0
2.5%
C11:0
2.5%
C12:0
5.0%
C13:0
2.5%
C14:0
2.5%
C14:1(
cis
-9)
1.5%
C15:0
1.5%
C16:0
10.0%
C16;1(
cis
-9)
5.0%
C17:0
2.5%
C18:0
5.0%
Compound
Qty. (%)
C18:1(
trans
)
2.5%
C18:1(
cis
)
15.0%
C18:2(
trans
)
2.5%
C18:2(
cis
)
10.0%
C18:3
5.0%
C20:0
2.5%
C20:1
1.5%
C20:5
2.5%
C22:0
2.5%
C22:1
1.5%
C22:6
2.5%
C23:0
1.5%
C24:0
2.5%
C24:1
2.5%
30mg/mL total in methylene chloride, 1mL/ampul
NLEA FAME Mix
(28 components)
ea.
35078
Compound
Qty. (%)
C4:0
4.0
C6:0
4.0
C8:0
4.0
C10:0
4.0
C11:0
2.0
C12:0
4.0
C13:
2.0
C14:0
4.0
C14:1(
cis
-9)
2.0
C15:0
2.0
C15:1(
cis
-10)
2.0
C16:0
6.0
C16;1(
cis
-9)
2.0
C17:0
2.0
C17:1(
cis
-10)
2.0
C18:0
4.0
C18:1(
trans
-9)
2.0
C18:1(
cis
-9)
4.0
C18:2(all-
trans
-9.12)
2.0
Compound
Qty. (%)
C18:2(all-
cis
-9,12)
2.0
C18:3(all-
cis
6,9,12)
2.0
C18:3(all-
cis
9,12,15)
2.0
C20:0
4.0
C20:1(
cis
-11)
2.0
C20:2(all-
cis
11,14,)
2.0
C20:3 (all-
cis
8,11,14)
2.0
C20:3(all-
cis
11,14,17)
2.0
C20:4(all-
cis
5,8,11,14)
2.0
C20:5(all-
cis
5,8,11,14,17)
2.0
C21:0
2.0
C22:0
4.0
C22:1(
cis
13)
2.0
C22:2(all-
cis
13,16)
2.0
22:6(all-
cis
4,7,10,13,16,19)
2.0
C23:0
2.0
C24:0
4.0
C24:1(
cis
-15)
2.0
30mg/mL total in
methylene chloride, 1mL/ampul
Food Industry FAME Mix
(37 components)
ea.
35077
Compound
Qty. (%)
methyl elaidate (C18:1
trans
-9)
10.0%
methyl linoleate (C18:2
cis
-9,12)
20.0%
methyl oleate (C18:1
cis
-9)
10.0%
methyl petroselinate (C18:1
cis
-6)
8.0%
methyl petroselaidate (C18:1
trans
-6)
8.0%
methyl stearate (C18:0)
20.0%
methyl transvaccenate (C18:1
trans
-11)
12.0%
methyl vaccenate (C18:1
cis
-11)
12.0%
10mg/mL total in methylene chloride, 1mL/ampul
cis
/
trans
FAME Mix
(8 components)
ea.
35079
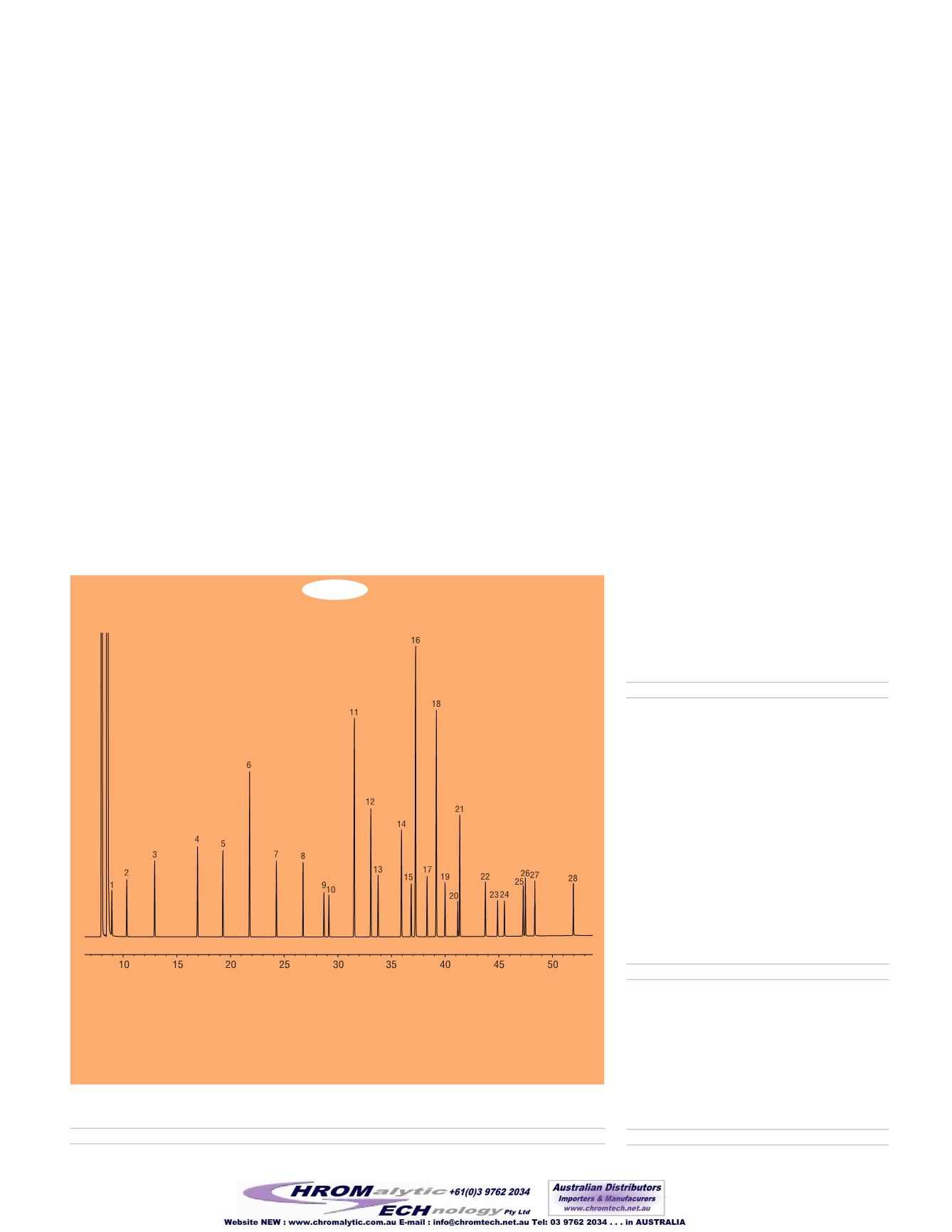
Figure 1
NLEA FAME Mix contains the components needed to standardize fat-by-fatty acid composition
methods, such as AOAC Method 996.06.
Column:
Rt-2560 100m, 0.25mm ID, 0.2µm (cat.# 13199)
Sample:
NLEA FAME Mix (cat.# 35078) 30mg/mL total FAMEs in
methylene chloride
Inj.:
1.0µL split (split ratio 100:1),
4mm inlet liner (cat.# 20814)
Inj. temp.:
225°C
Carrier gas:
hydrogen, constant flow
Flow rate:
1.2 mL/min.
Oven temp.:
100°C (4 min. hold) to 240°C
@ 3°C/min. (10 min. hold)
Det.:
FID @ 250°C
1. C4:0 methyl butyrate
2. C6:0 methyl heanoate
3. C8:0 methyl octanoate
4. C10:0 methyl decanoate
5. C11:0 methyl undecanoate
6. C12:0 methyl laurate
7. C13:0 methyl tridecanoate
8. C14:0 methyl myristate
9. C14:1 methyl myristoleate (
cis
-9)
10. C15:0 methyl pentadecanoate
11. C16:0 methyl palmitate
12. C16:1 methyl palmitoleate (
cis
-9)
13. C17:0 methyl heptadecanoate
14. C18:0 methyl stearate
15. C18:1 methyl elaidate (
trans
-9)
16. C18:1 methyl oleate (
cis
-9)
17. C18:2 methyl linoelaidate (
trans
-9,12)
18. C18:2 methyl linoleate (
cis
-9,12)
19. C20:0 methyl arachidate
20. C20:1 methyl eicosenoate (
cis
-11)
21. C18:3 methyl linolenate (
cis
-9,12,15)
22. C22:0 methyl behenate
23. C22:1 methyl erucate (
cis
-13)
24. C23:0 methyl tricosanoate
25. C24:0 methyl lignocerate
26. C20:5 methyl eicosapentaenoate
(
cis
-5,8,11,14,17)
27. C24:1 methyl mervonate (
cis
-15)
28. C22:6 methyl docosahexaenoate
(
cis
-4,7,10,13,16,19)
GC_FF00651


















