DET
innovations in chemical detection
TID-3-N
2 {
°
2 ) :
selective for VOLATILE HALOGENATES
Equipment:
This detection mode is similar to the TIO-'-N
2
mode
except aTID-3 type source is used. The T10-3 source
can be mounted in either a TID/FID, REMOTE FID,
FTID, PTID, or TANDEM TID tower. Detector gas is N
2
through the lines which normally
provideH,"
and air
to an FlOor NPO. (Air or O
2
are other possible choices
for the detector gases.) The source is heated by a
constant current supply and is polarized at -45 Volts
relative to the collector. In most applications, the
surface temperature of the source is in the range of
600-800
o
Cwhich produces a visible orange glow.
Principle:
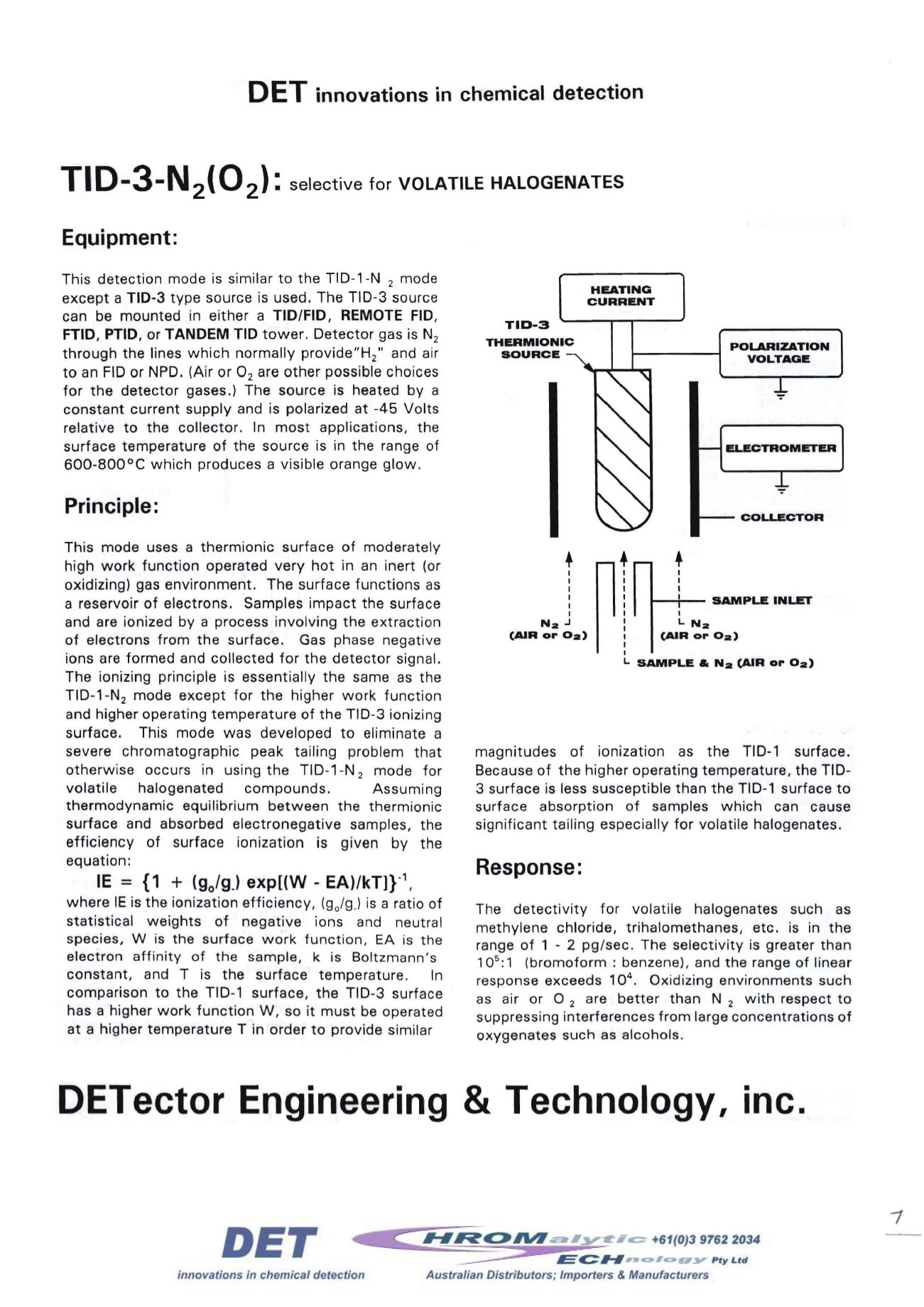
TID-3
THERMIONIC
SOURCE
HEATING
CURRENT
POLARIZATION
VOLTAGE
--' ELECTROMETER
__-- COLLECTOR
Response:
magnitudes of ionization as the TIO-'
surface.
Because of the higher operating temperature, the TIO–
3 surface is less susceptible than the TIO-' surface to
surface absorption of samples which can cause
significant tailing especially for volatile halogenates.
The detectivity for volatile halogenates such as
methylene chloride, trihalomethanes, etc. is in the
range of , - 2 pg/sec. The selectivity is greater than
lOs: 1
(bromoform: benzene), and the range of linear
response exceeds
10
4
•
Oxidizing environments such
as air or
°
2
are better than N
2
with respect to
suppressing interferences from large concentrations of
oxygenates such as alcohols.
1--+-
SAMPLE INLET
I
L.
N
z
(AIR or Oz)
r
1
L.
SAMPLE .. Na (AIR or Oz)
I
I
I
I
I
I
Nz
.J
(AIR or Oz)
This mode uses a thermionic surface of moderately
high work function operated very hot in an inert (or
oxidizing) gas environment. The surface functions as
a reservoir of electrons. Samples impact the surface
and are ionized by a process involving the extraction
of electrons from the surface. Gas phase negative
ions are formed and collected for the detector signal.
The ionizing principle is essentially the same as the
TI0-,-N
2
mode except for the higher work function
and higher operating temperature of the T10-3 ionizing
surface. This mode was developed to eliminate a
severe chromatographic peak tailing problem that
otherwise occurs in using the TIO-'-N
2
mode for
volatile halogenated compounds.
Assuming
thermodynamic equilibrium between the thermionic
surface and absorbed electronegative samples, the
efficiency of surface ionization is given
by
the
equation:
IE
=
{1
+
(go/g.)
exp[(W - EA)/kTl}",
where IEis the ionization efficiency, (go/gJ is a ratio of
statistical weights of negative ions and neutral
spec ies, W is the surface work function, EA is the
electron affinity of the sample, k is Boltzmann's
constant, and T is the surface temperature.
In
comparison to the TIO-, surface, the T10-3 surface
has a higher work function W, so it must be operated
at a higher temperature T in order to provide similar
DETector Engineering
&
Technology, inc.


