DET
innovations in chemical detection
TID-1 -N
2(
0
2) :
selective for
NITRO
I
OXYGENATED
I
or
HALOGENATED
compounds
Equipment:
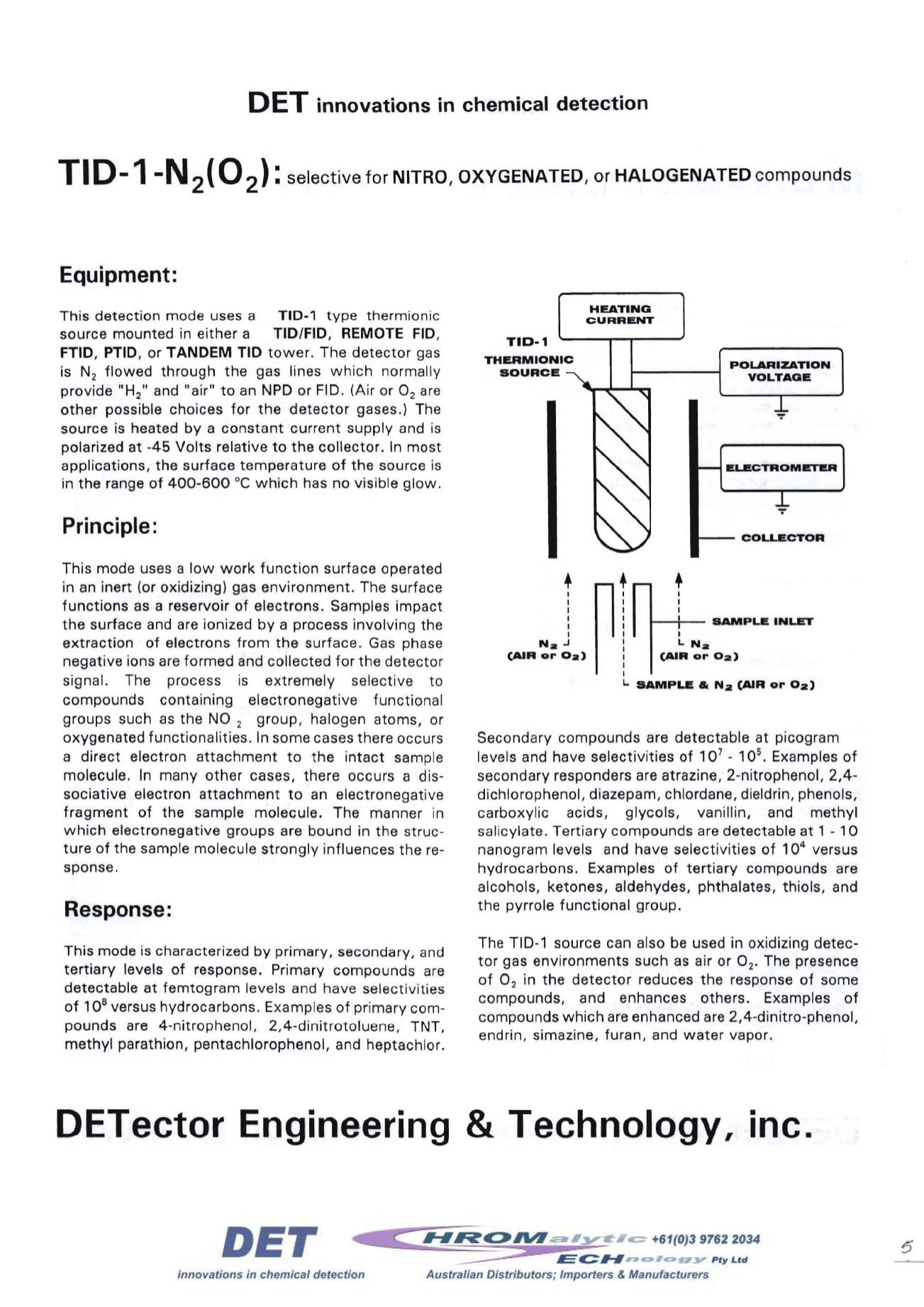
This detection mode uses a
TID-1
type thermionic
source mounted in either a TID/FID,
REMOTE
FID,
FTID, PTID, or
TANDEM TID
tower. The detector gas
is N
2
flowed through the gas lines which normally
provide "H
2 "
and "air" to an NPD or FlO. (Air or O
2
are
other possible choices for the detector gases.) The
source is heated by a constant current supply and is
polarized at -45 Volts relative to the collector. In most
applications, the surface temperature of the source is
in the range of 400-600 °Cwhich has no visible glow.
Principle:
TID-1
THERMIONIC
SOURCE
HEATING
CURRENT
POLARIZATION
VOLTAGE
ELECTROMETER
COLLECTOR
Secondary compounds are detectable at picogram
levels and have selectivities of 10
7
-
10
5
•
Examples of
secondary responders are atrazine, 2-nitrophenol, 2,4–
dichlorophenol, diazepam, chlordane, dieldrin, phenols,
carboxylic acids, glycols, vanillin, and methyl
salicylate. Tertiary compounds are detectable at 1 - 10
nanogram levels and have selectivities of 10
4
versus
hydrocarbons. Examples of tertiary compounds are
alcohols, ketones, aldehydes, phthalates, thiols, and
the pyrrole functional group.
This mode uses a low work function surface operated
in an inert (or oxidizing) gas environment. The surface
functions as a reservoir of electrons. Samples impact
the surface and are ionized by a process involving the
extraction of electrons from the surface. Gas phase
negative ions are formed and collected for the detector
signal. The process is extremely selective to
compounds containing electronegative functional
groups such as the NO
2
group, halogen atoms, or
oxygenated functionalities. In some cases there occurs
a direct electron attachment to the intact sample
molecule. In many other cases, there occurs a dis–
sociative electron attachment to an electronegative
fragment of the sample molecule. The manner in
which electronegative groups are bound in the struc–
ture of the sample molecule strongly influences the re–
sponse.
Response:
•
I
I
I
I
I
I
N
a
..J
(AIR or Oa)
1--+-
SAMPLE INLET
I
L.
Na
(AIR or Oa)
L.
SAMPLE & N
a
(AIR
or
0a)
This mode is characterized by primary, secondary, and
tertiary levels of response. Primary compounds are
detectable at femtogram levels and have selectivities
of 10
8
versus hydrocarbons. Examples of primary com–
pounds are 4-nitrophenol. 2,4-dinitrotoluene, TNT,
methyl parathion, pentachlorophenol, and heptachlor.
The T10-1 source can also be used in oxidizing detec–
tor gas environments such as air or
02'
The presence
of O
2
in the detector reduces the response of some
compounds, and enhances others. Examples of
compoundswhich areenhanced are 2,4-dinitro-phenol,
endrin, simazine, furan, and water vapor.
DETector Engineering
&
Technology,
•
Inc.


