
•
14
•
2007.01
Clinical/Forensics
Analyze and ConfirmCannabinoids by LC/MS/MS
Using an Allure® Biphenyl Column
by Kristi Sellers, Clinical/Forensic Innovations Chemist, Becky Wittrig, Ph.D., HPLC Product Marketing Manager,
and André Schreiber, Ph.D., Application Chemist, Applied Biosystems
As marijuana is smoked, the main psychoactive
component,
Δ
9
-tetrahydrocannabinol (
Δ
9
-THC), is
quickly absorbed and metabolized to 11-hydroxy-
Δ
9
-tetrahydrocannabinol (hydroxy-THC), an
active metabolite. Hydroxy-THC is further metab-
olized, rapidly, to 11-nor-9-carboxy-
Δ
9
-tetrahydro-
cannabinol (carboxy-THC), an inactive metabolite
commonly found in urine, blood, hair, and tissues.
1
GC/MS often is used for confirming and quantify-
ing
Δ
9
-THC and carboxy-THC
2
; however, GC/MS
methods require time-consuming steps, like
derivatization, to obtain acceptable chromatogra-
phy. By using HPLC, derivatization can be elimi-
nated, saving time without sacrificing sensitivity.
We developed a quantitative method for analyzing
underivatized cannabinoids by HPLC/tandem
mass spectrometry. Our goals were threefold; 1) to
optimize column selection, 2) to provide a short
analysis time, and 3) to obtain reliable confirmation
and quantification data in the low nanogram range
(< 10ng). We used an Applied Biosystems API 3200
MS/MS detector coupled to a Shimadzu LC20AD
Prominence Series chromatograph for optimum
chromatographic and detection capabilities.
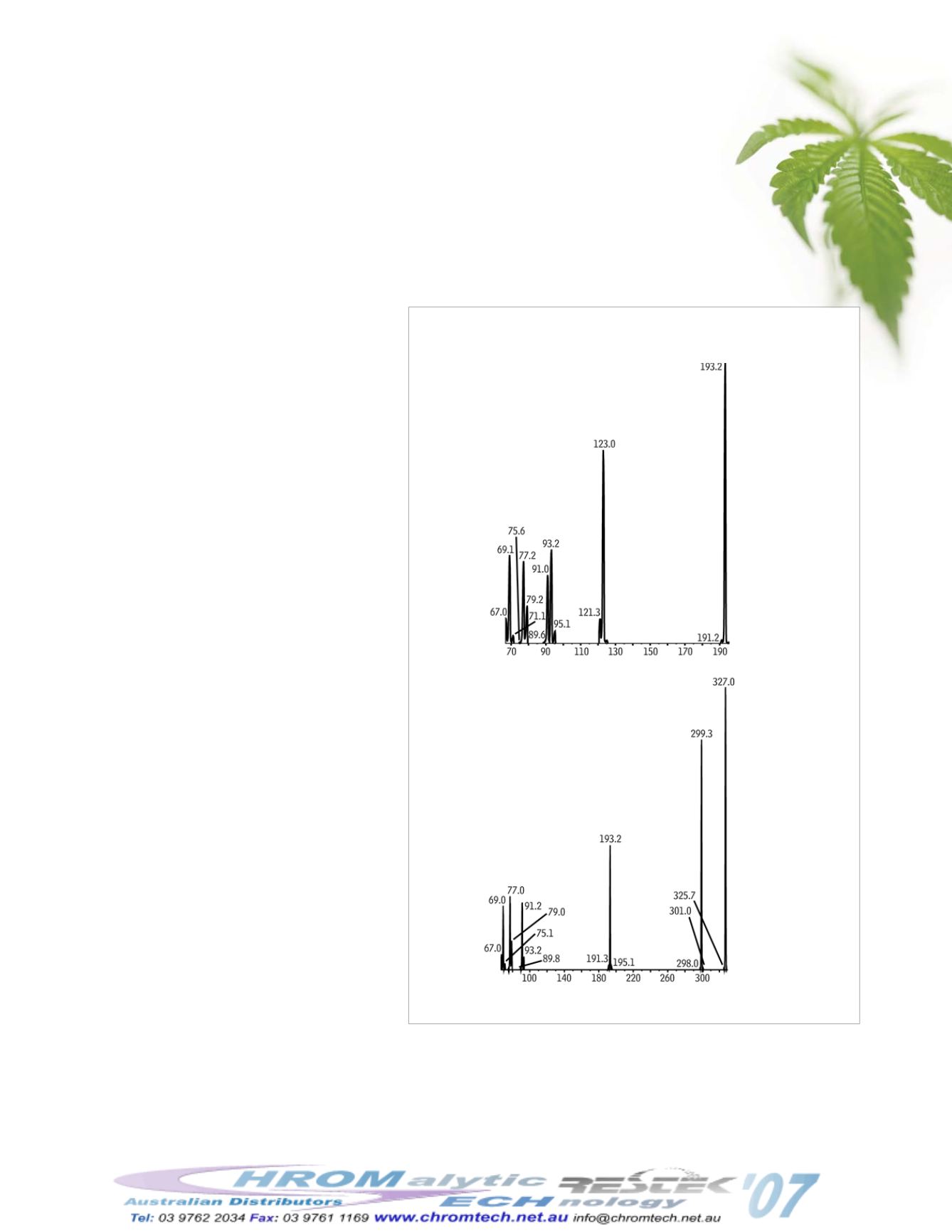
Figure 1 shows the final product spectra for
Δ
9
-
THC and carboxy-THC used to develop the
+MRM (multiple reaction monitoring) method.
3
We determined the 30mm, 2.1mmID, 3µm Allure®
Biphenyl HPLC column to be the best column for
this analysis. This column employs a unique sepa-
ration mechanism,
π
-
π
interaction, which greatly
improves selectivity and retention, relative to con-
ventional C18 phases. In addition, with the
increased retention of the biphenyl phase, higher
amounts of methanol can be used in the mobile
phase. This noticeably increases sensitivity when
using an electrospray interface.
The Allure® Biphenyl column provides good reso-
lution of all compounds in less than 5 minutes –
including baseline resolution of
Δ
9
-THC and
cannabidiol, which have very similar product ion
spectra and +MRM transitions (Figure 2). By using
MS/MS detection, we were able to target two
Figure 1
Final product spectra used in developing MRM
transitions for compound identification and optimized sensitivity.
Δ
9
-THC
Carboxy-THC
• Faster sample throughput (short analysis time, no derivatization)
• Reliable response at 1ng on-column
• Undisputable identification, using two +MRM transitions
For conditions see Figure 2.
LC_PH0422











