
•
6
•
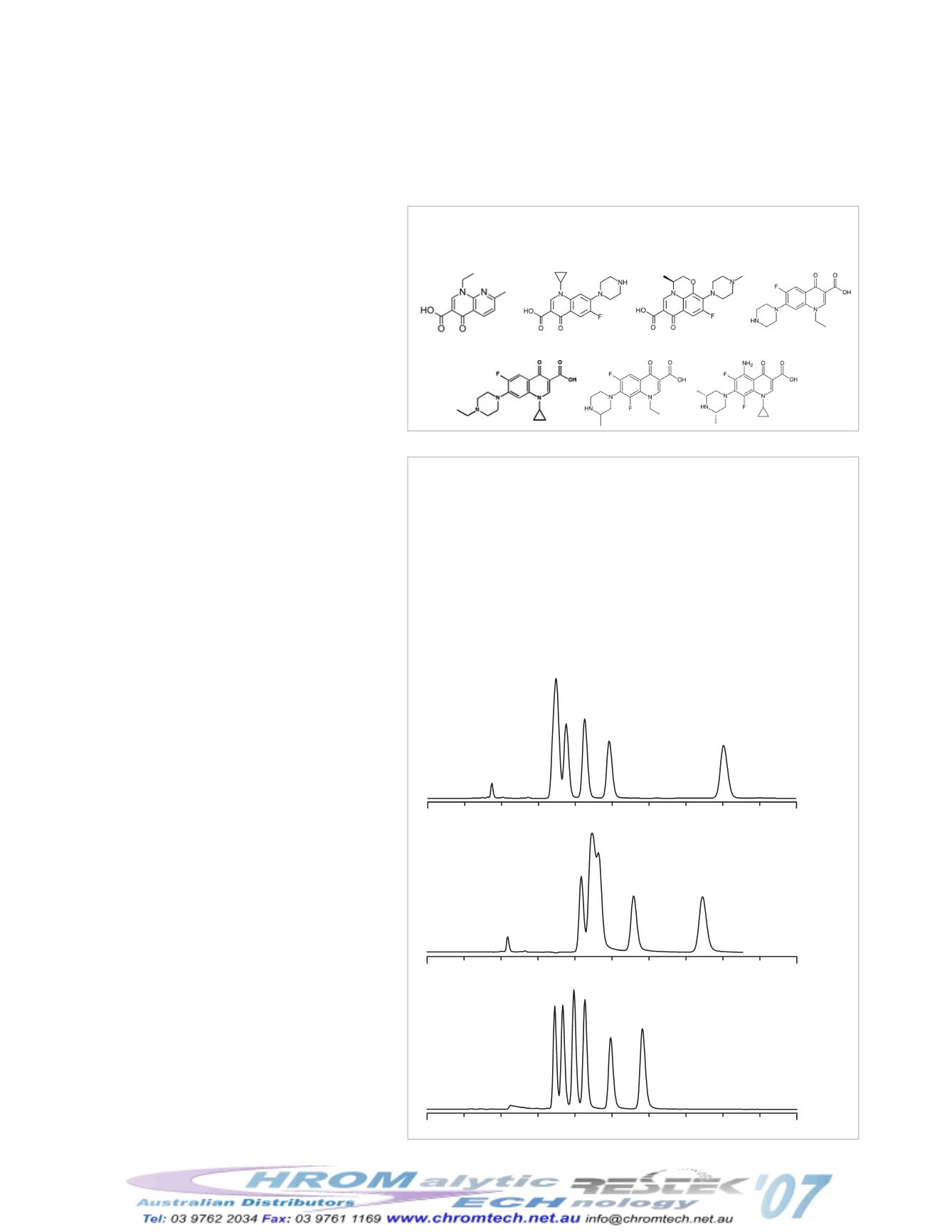
Figure 2
Greater retention capabilities and better selectivity
enable you to use simple two-component mobile phases with an
Allure® PFP Propyl column.
2007.01
Foods, Flavors & Fragrances
Simplified LC/MS/MS Analysis of Fluoroquinolones
Using An Allure® PFP Propyl Column
By Rick Lake, Pharmaceutical Innovations Chemist, and Benjamin Smith, Applications Technician
Fluoroquinolones are broad-spectrum antibiotics,
used in both human and veterinary medicine.
Because they are widely used, fluoroquinolones are
target compounds in many analysis sectors, from
research and clinical testing to environmental
impact and residues in food. We have determined
that an Allure® PFP Propyl column offers good
retention capacity, and better selectivity than a
C18 column, allowing simple method develop-
ment strategies for fluoroquinolones.
Parent compound nalidixic acid is the structural
basis for all quinolones, and fluoroquinolones are
a fluorine-containing subset of this group (Figure
1). Chemically, fluoroquinolones exhibit ampho-
teric behavior: the nalidixic acid portion of the
molecule has acidic functionality (carboxylic
acid), while the compound as a whole also express-
es a basic functionality. These characteristics, and
the typical presence of polar functional groups,
make chromatographic retention of the com-
pounds difficult when using an alkyl phase and a
simple (two-component) mobile phase. Polar
groups reduce retention on alkyl phases, making a
highly aqueous mobile phase, or ion-pairing, nec-
essary for acceptable retention.
For non-selective, non-MS analyses, like potency
assays, fluoroquinolones traditionally have been
analyzed by reversed phase HPLC, on a C18 phase
and in a highly aqueous mobile phase, as described
in the USP monograph for ciprofloxacin.
1
When
mass spectrometry is dictated, and a highly aque-
ous mobile phase is undesirable, ion-pairing with
a volatile “MS friendly” reagent, like nonafluo-
ropentanoic acid, has been used to increase reten-
tion. Although these mechanisms are sufficient, we
sought to determine if, with a simple mobile
phase, an Allure® PFP Propyl column would offer
better retention, and possibly better selectivity,
than a C18 phase.
Initially, we assayed the analytes on a C18 column, in
an aqueous buffer and acetonitrile, to evaluate the
retention and selectivity that could be achieved with
a conventional stationary phase and isocratic mobile
phase. As expected, retention was poor: an accept-
able retention capacity value (roughly 2-5) required
an aqueous concentration of 80% (Figure 2). Next,
to see if we could improve retention through ionic
• Increase retention without ion-pairing.
• Better selectivity than C18 or cyano phases.
• Use desirable high-organic mobile phases
for better ESI LC/MS sensitivity.
Figure 1
The polarity of fluoroquinolones make
them a challenge to retain on C18 phases.
Nalidixic acid
Ciprofloxacin
Levofloxacin
Norfloxacin
Enrofloxacin
Lomefloxacin
Sparfloxacin
1. norfloxacin
2. levofloxacin
3. ciprofloxacin
4. lomefloxacin
5. enrofloxacin
6. sparfloxacin
Sample:
Inj.:
5µL
Conc.:
~50µg/mL each component
Sample diluent: mobile phase
Column:
Dimensions:
150 x 4.6 mm
Particle size: 5µm
Pore size:
60Å
Conditions:
Mobile phase: 10mM potassium phosphate monobasic (pH 2.5):
acetonitrile, 40:60 (v:v) Allure
®
PFP Propyl
or 80:20 (v:v) C18, Cyanopropyl
Flow:
1.0mL/min.
Temp.:
ambient
Det.:
UV @ 220nm
0
2
4
6
8
10
1 3
2
4
5
6
1
3 4
5
6
4
5
6
2,3
1,2
Excellent retention
and better selectivity
C18
20% ACN
20% ACN
60% ACN
Cyanopropyl
Allure® PFP Propyl











