
•
3
•
2005 vol. 2
The United States Environmental Protection
Agency (US EPA) is actively developing regula-
tions, limits, and control measures for monitoring
and controlling mercury emissions from coal-fired
power generators—one of the major sources of
mercury emissions into the environment.¹ As these
regulations and guidelines are developed and
implemented, proper equipment will be needed
for accurate sampling and analysis. Testing costs
for mercury can be substantial (Table 1)², so inac-
curate analyses can have financial as well as envi-
ronmental repercussions.
In flue streams from coal-fired power generators,
mercury exists in three forms: elemental, the +2
oxidation state (Hg
++
), and attached to particulate
matter. Hg
++
often reacts with sulfur compounds,
nitrogen, chlorine, and/or oxygen, to produce sul-
furous, nitrous, chloride, and oxide mercury
species. Elemental and oxidized mercury can easily be lost to reactions and
adsorption on the inner surfaces of monitoring equipment. In order to accu-
rately sample and quantify mercury in all forms, it is important to use inert
sample pathways. Laboratory testing and field results have proven that
Sulfinert® treated sampling and testing equipment is essentially inert to active
molecules³, including mercury.
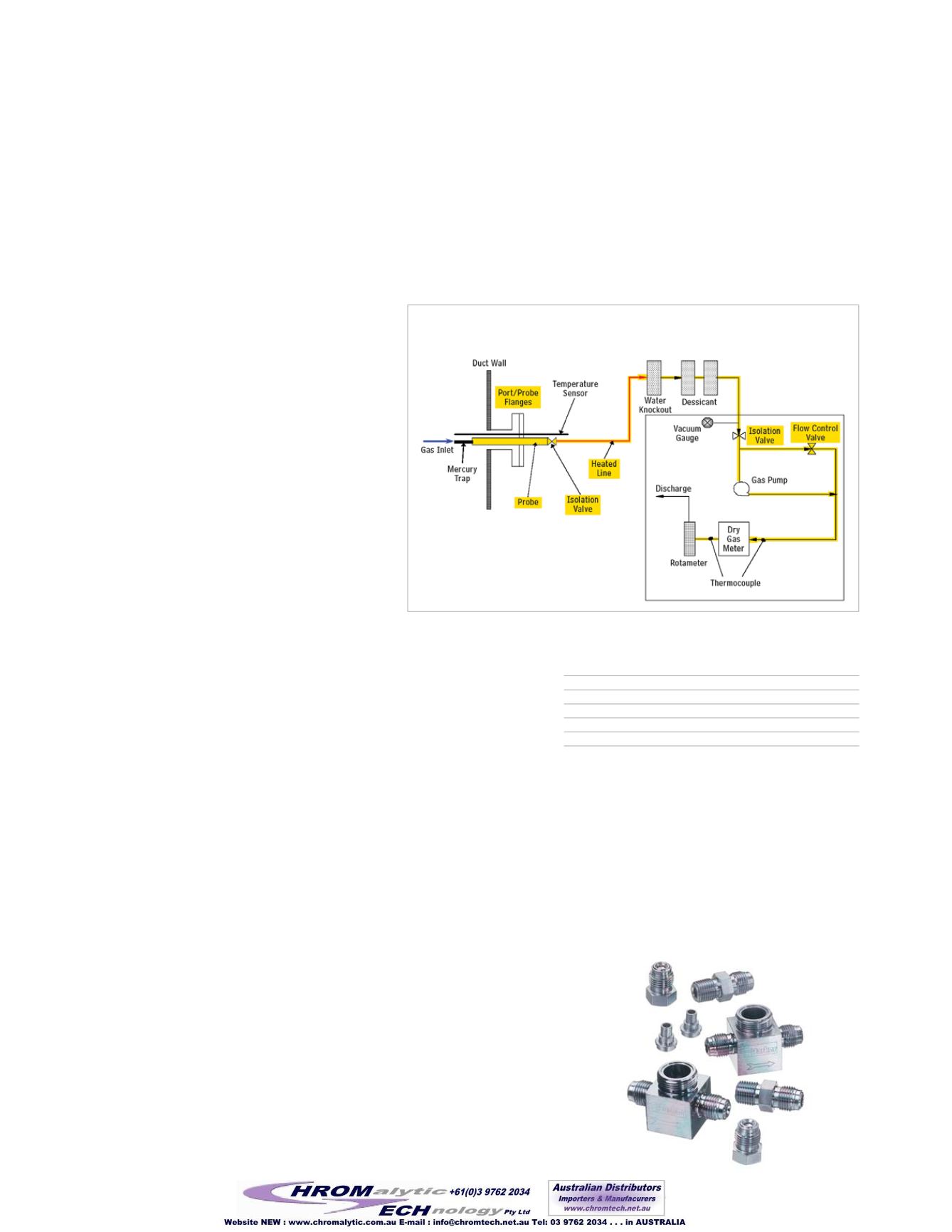
Siltek®/Sulfinert® treatment can be applied to many of the components in a
mercury sampling stream, including probe tubing, impingers, fittings, filters,
housings, and transfer tubing (Figure 1). Treating all of the components of a
stack or continuous emission monitoring system will greatly improve analyt-
ical reliability and sensitivity, which will be needed as regulations are brought
on line and emission quotas are enforced. Fast and accurate testing, without
re-work, can save a great deal of time and money.
Similarly, a Siltek®/Sulfinert® treated sampling system will improve the relia-
bility of data for sulfurous oxides and nitrous oxides (SO
x
and NO
x
). As with
mercury, it is difficult to reliably transfer these compounds through untreated
sampling equipment.
In addition to preventing adsorption of reactive compounds,
Siltek®/Sulfinert® treatment will act as a barrier, protecting and prolonging
the lifetime of treated equipment. The durable layer will withstand tempera-
tures to 400°C.
We offer Siltek®/Sulfinert® treated tubing, sample cylinders, and other com-
ponents from stock; to discuss custom treatment of system components,
please contact the Restek Performance Coatings team.
Accurately Monitor Mercury-Sulfur-Nitrogen Compounds
Siltek®/Sulfinert® Treatment Prevents Adsorption of Mercury, Sulfur Oxides,
or Nitrous Oxides in Emission Monitoring Equipment
By Gary Barone, Restek Performance Coatings Division Manager, David Smith, RPC Chief Scientist, and Martin Higgins, RPC Chief Engineer
• Improved analytical reliability and sensitivity for mercury, SO
x
, or NO
x
compounds.
• Protection from corrosion—longer component lifetime.
• Apply to new or existing equipment.
Table I
Typical costs of mercury sampling (U.S.).²
Method
Approx. Cost of Analysis
US EPA 29
$300
US EPA 101A
$100
ASTM D6784-02
$250
US EPA 324
$430
FAMS
$640
Figure 1
Highlighted components of a mercury sampling train,
4
and all tubing in the system, can be Siltek®/Sulfinert® treated.
Restek offers treated and untreated tubing, fittings, and valves, passive air sampling kits, air sampling canisters
and miniature air canisters, sample loops, and more. For more information, request our catalog or visit us
online.
www.restekcoatings.comReferences
1. Pottinger, M., S. Stecklow, and J.J. Fialka,
Invisible Export, A Hidden
Cost of China’s Growth: Mercury Migration
The Wall Street Journal
Online, Dec. 17, 2004.
2. Serne, J.C.,
An Overview and Comparison of Available Mercury
Emission Test Methods for Boilers
Symposium on Air Quality
Measurement; Methods and Technology 2005, San Francisco, CA; Air
& Waste Management Association. paper no. 439, pg. 9.
3. Barone, G., M. Higgins, D. Smith, S. Rowan, W.J. Gross, and P. Harris,
The Surface for Sulfurs
Hydrocarbon Engineering, Dec. 2004, pp 47-
50.
4. Proposed Method 324.
Determination of Vapor Phase Flue Gas
Mercury Emissions from Stationary Sources Using Dry Sorbent Trap
Sampling
United States Environmental Protection Agency. Washington,
D.C. p. 5.


















