RestekCorporation • (800) 356-1688 • (814)353-1300 •
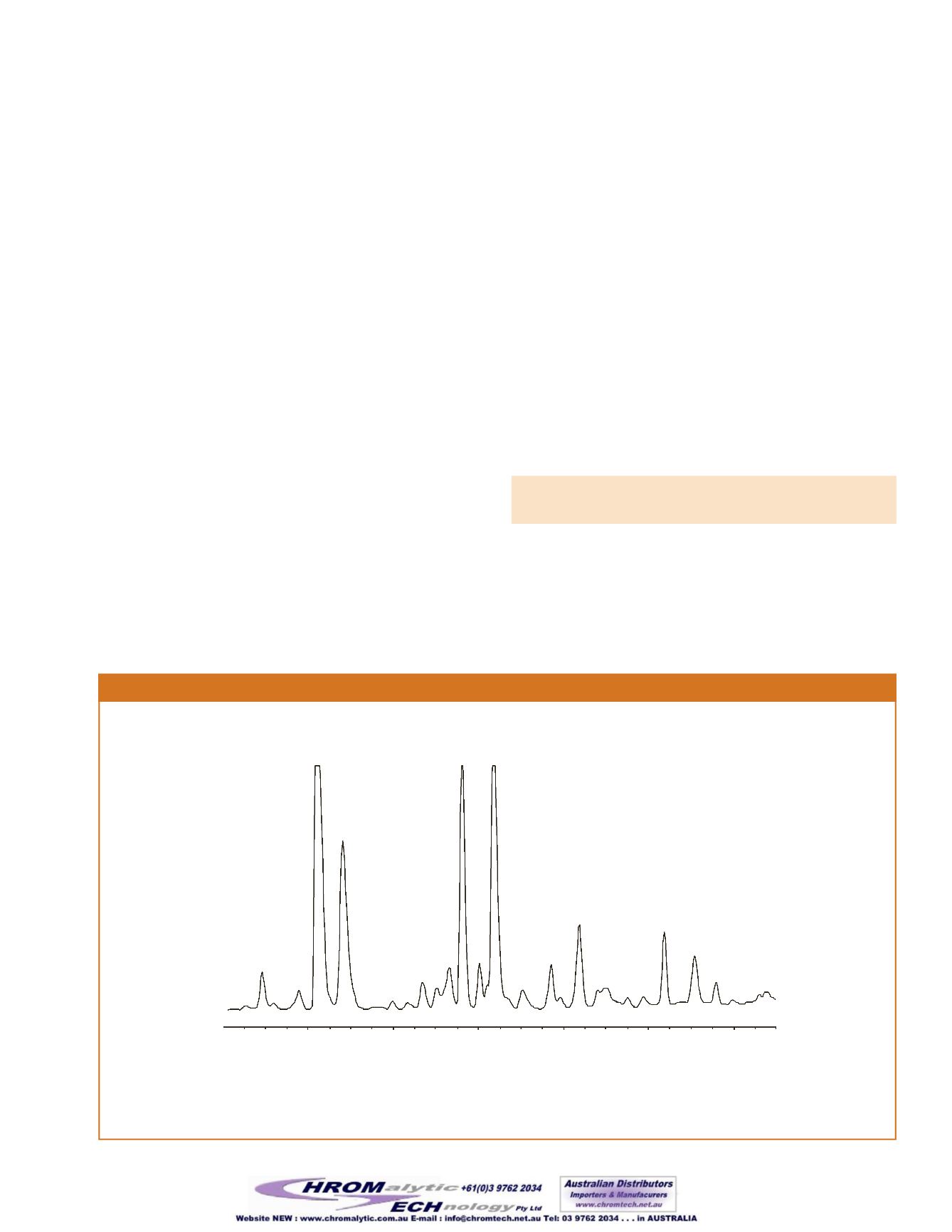
Figure4
The 0.25mm IDRt-
g
DEXsa
™
column provides detection of benzaldehyde, andmethyl-and ethyl-3-hydroxybutyrate.
30m, 0.25mm ID, 0.25µmRt-
g
DEXsa
™
(cat.# 13113) 10µL splitless injection
of a grape-flavored drink
.
Oven temp.:
40°C (hold 1min.) to 200°C
@ 2°C/min.;
Inj. / det. temp.:
200°C/230°C;
Carrier gas:
He;
Linear velocity:
35cm/sec. set@ 40°C;
Splitless hold time:
1min.
min. 28
30
32
34
36
38
1. (S)-(+)-methyl-3-hydroxybutyrate
2. (R)-(–)-methyl-3-hydroxybutyrate
3. benzaldehyde
4. (S)-(+)-ethyl-3-hydroxybutyrate
5. (R)-(–)-ethyl-3-hydroxybutyrate
1
2 4
5
40
3
ComparingGrapeJuicesandGrape-Flavored
Beverages
ExtractionProcedure
Grape juice,whitegrape juice, grapedrink, grape-flavored soda,
andagrape-flavored sport drinkwereevaluated. Each16–20oz.
beveragewas added to a500mL separatory funnel. Thirty
milliliters ofmethylene chloridewere added to the sample in the
separatory funnel,whichwas shaken for 3–5minutes. The
extractwas then collected into abeaker.This procedurewas
repeated three times. Theorganic extractwas then funneled
through anhydrous sodium sulfate to eliminatewater and
transferred to aKuderna-Danish collectorwith aSnyder column.
Thiswas immersed into a hotwater bathof 65°Cuntil the extract
was concentrated to4mL.
Analysis
Tenmicroliters of samplewas introducedviadirect injection.A
1.5mx0.53mmguard columnwas connected to the4mmopen-
topUniliner
®
sleeve and to the30m, 0.32mm ID, 0.25µm
Rt-
g
DEXsa
™
column, to accommodate the largevolume injection
and toprotect the analytical column. Some spectral confirmation
was conductedbyGC/MSon a30m, 0.25mm ID, 0.25µm
Rt-
g
DEXsa
™
column, using splitless analysis.
Results
Themethyl-3-hydroxybutyratewas essentially racemic inboth
thegrape andwhitegrape juices, as shown in
Figure3a
and
3b
.
Itwas also racemic inonegrape-flavoreddrink, but atmuch
lower concentrations.Analysis on a0.25mm IDRt-
g
DEXsa
™
columnwith a slower linear velocityprovides resolutionof
benzaldehydeand (R)-methyl-3-hydroxybutyrate (
Figure4
).The
(R)-ethyl 3-hydroxybutyratewaspredominant inboth juices and
grape-flavoreddrink.Neither chiral compoundwasdetected in
thegrape-flavored sodaor sport drink.
Beta-cyclodextrinphases can separate avarietyof chiral
indicating compounds in flavors, but arenot effective in all
applications.Usingdifferent cyclodextrinderivativescanhelp
chiral selectivity, but going to a larger cyclodextrin sometimes is
necessary. Switching froma2,3-di-O-methyl-6-O-
tert
-
butyldimethylsilyl-
b
-cyclodextrin toa2,3-di-O-acetyl-6-O-
tert
-
butyldimethylsilyl-
b
-cylcodextrincolumnpartially improved
enantiomer separation for chiral indicating compounds ingrape
flavor.However, the2,3-di-O-acetyl-6-O-
tert
-butyldimethylsilyl-
g
-cyclodextrincolumnprovided thebest enantiomericprofileof
these compounds.TheRt-
g
DEXsa
™
column allows detectionof
racemicmethyl-3-hydroxybutyrateand (R)-ethyl-3-
hydroxybutyrate in juices anddrinks that contain authenticgrape
flavor. This column canoffer certain separations that cannot be
achievedbybeta-cyclodextrincolumns andmaybe amore
suitable alternative for your chiral analysis.
References
1. Dr. Joulain,RobertetCorp., “privatecommunication.”


