SelectingaGCColumn
Strategic column choices can improve lab productivity by assuring that speed and
resolution are optimized. While the number of choices available can be daunting,
consideration of the resolution equation variables—selectivity, retention (capacity),
andefficiency—simplifies thedecision.Selectivitydetermineswhich stationaryphase is
most appropriate, and it canbe approximatedusing retention indicesor existingappli-
cations.Once the phase has been chosen, physical dimensions (internal diameter, film
thickness, length)canbe selectedbasedon retentionandefficiency.Understandinghow
selectivity, retention, and efficiency influence separations allows analysts to make
effective, informed choices andquickly select the best column for specific separations.
23
GCCOLUMNS |
CAPILLARYCOLUMNS
Selecting a GC Column
Retention
Selectivity
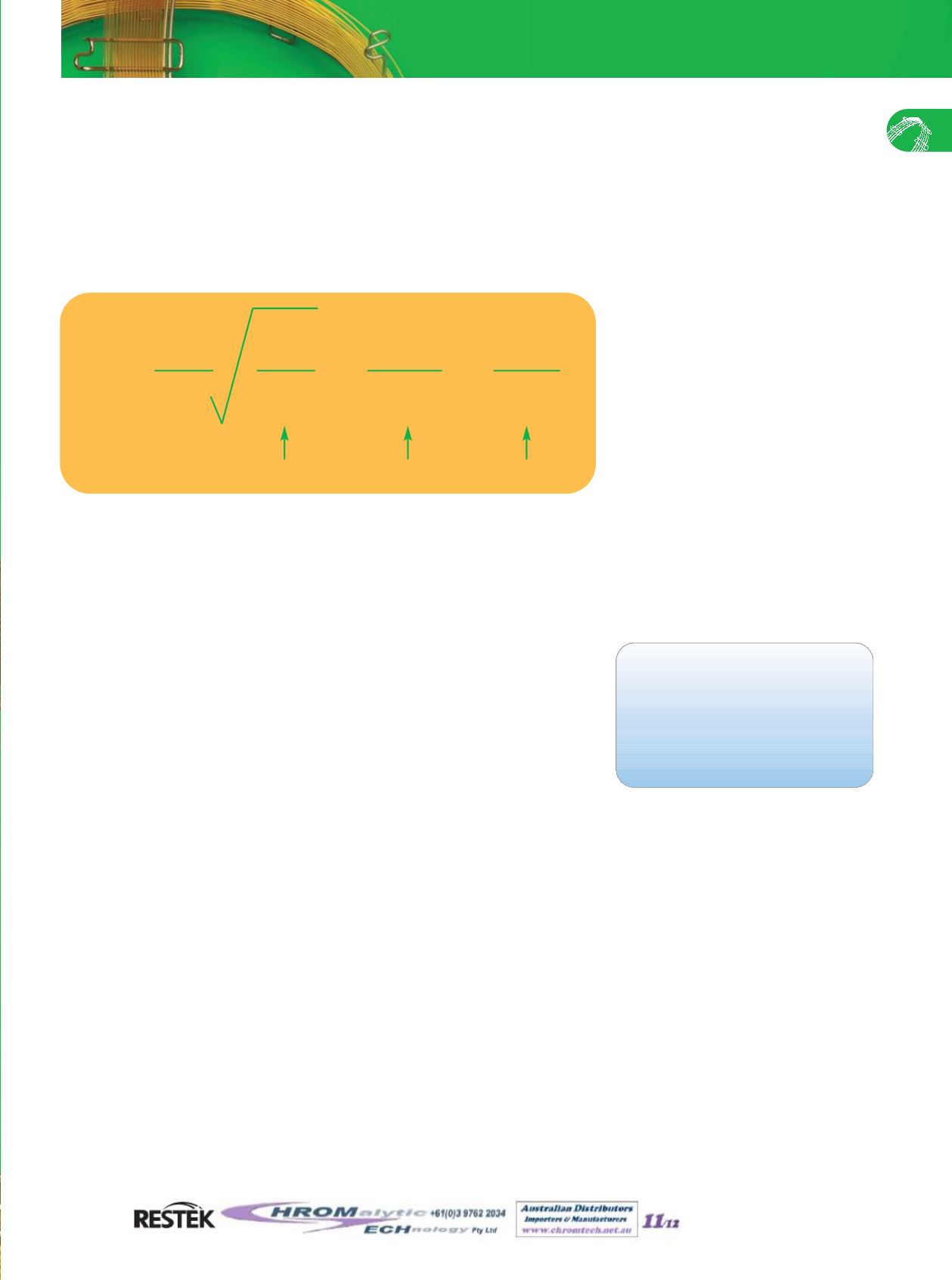
R= resolution
L=column length
H=HETP
k=capacity factor
α
= selectivity
R=
X X
1 L k
α
-1
4 H k+1
α
Efficiency
Chroma
BLOG
raphy
Topical and timely insights from top
chromatographers.
Visit us at
blog.restek.com
Selectivity,
α
The selectivity of the capillary column is directly related to how the analytemolecule
interacts with the stationary phase being considered. If the analyte strongly interacts
with the stationary phase, it can be said that strong intermolecular forces exist. These
intermolecular forces of attraction between the analyte and the stationary phase are a
functionof the structureof both theanalytemoleculeand the stationaryphase. If these
two structures are similar, then the attractive forces are strong. If they are dissimilar,
then analyte to stationary phase attraction is weak, and less retention is observed.
Therefore,when selectinga stationaryphase, knowledgeof the structureof the analytes
of interest and the stationary phase is crucial. The reference table on page 27 provides
the chemical structure of Restek’smost common stationaryphases.
An example of selectivity can be shown using benzene and butanol (both have nearly
the same boiling point) eluting through the 20% diphenyl/80% dimethyl polysiloxane
stationary phase (Rtx®-20). The benzene molecule will dissolve into the stationary
phase more readily than the butanol based on the concept that “likes dissolve likes”.
Since benzene solvatesmore readilywith the stationary phase, it hasmore interactions
with the stationary phase as it elutes through the column. Therefore, the elutionorder
of these two compounds on the Rtx®-20 stationary phase will be butanol first and
benzene second.
Asmethyl groupsare replacedbydifferent functionalities suchasphenylorcyanopropyl
pendant groups, the selectivity of the column shifts towards compounds that will have
a better solubility in the stationary phase. For example the Rtx®-200 stationary phase
provides high selectivity for analytes containing lone pair electrons, such as halogens,
nitrogen, or carbonyl groups. Polyethylene glycol columns, such as the Stabilwax® and
Rtx®-Wax columns are highly selective towards polar compounds such as alcohols.
Againusing the example above, thebutanolmore readily solvates into thepolyethylene
glycol stationaryphase; therefore, thebutanolwill havemore interactionwith thephase
and elute after benzene.
Website :
E-mail :
TelNo : 03 9762 2034 . . . inAUSTRALIA
Mar 2011


