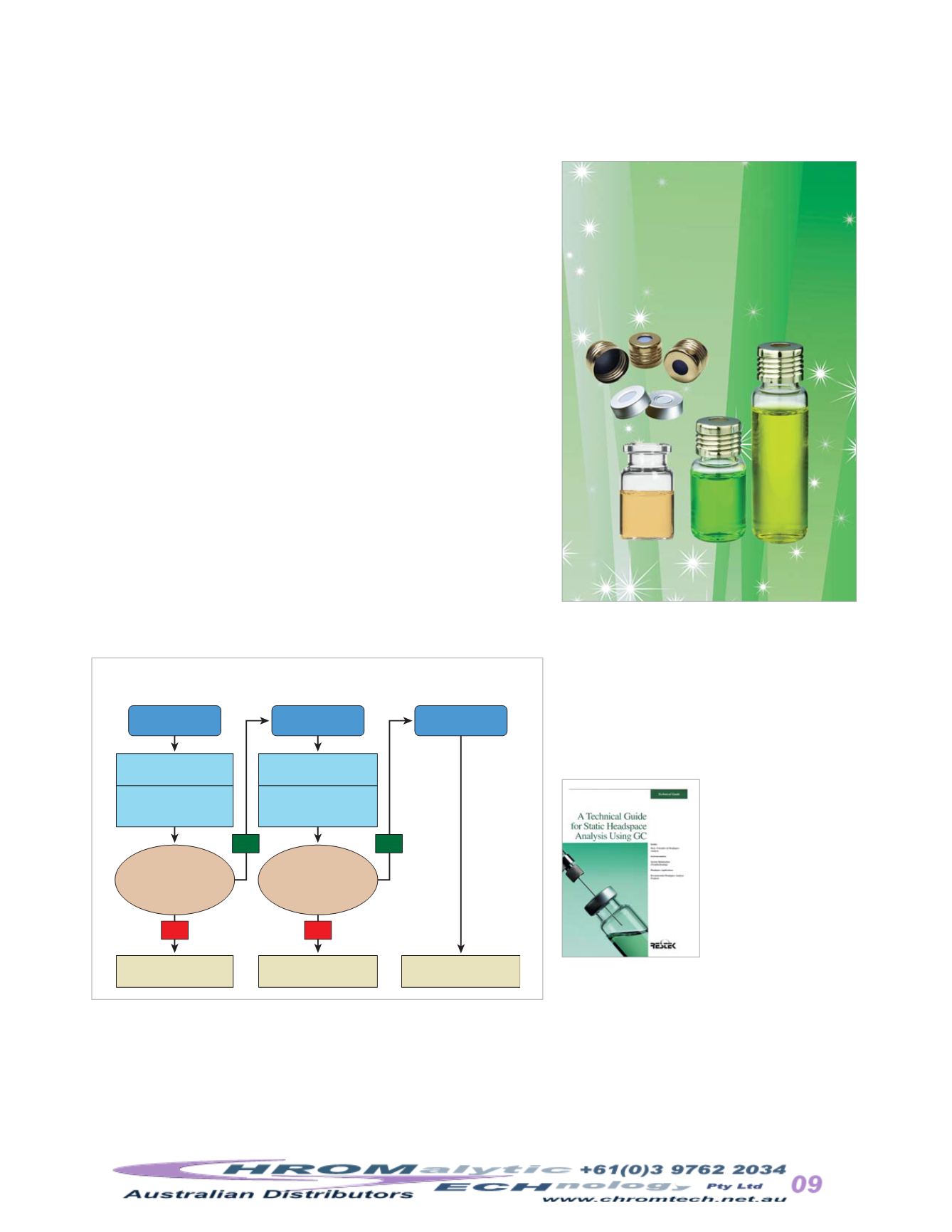
Figure3
Analytical flowchart for residual solvent testingunder the
revisedUSP<467>method.
ProcedureA
Identification
ProcedureB
Confirmation
ProcedureC
Quantification
PrepareStandard
andTest Solutions
PerformProcedureUnder
Method-SpecifiedSystem
andConditions
Residual Solvents
PeaksPresent at an
AreaGreater than the
Corresponding
Standard?
NO
NO
PassesTest
NoFurtherAction
YES
YES
PrepareStandard
andTest Solutions
PerformProcedureUnder
Method-SpecifiedSystem
andConditions
Residual Solvents
PeaksPresent at an
AreaGreater than the
Corresponding
Standard?
PassesTest
NoFurtherAction
CalculateAmountof
Residual SolventsPresent
AchievingUSP<467>Compliance
YourGuide toSuccessfully Implementing theRevisedMethod
•
3
•
Restekcarriesa full
lineofheadspace
essentials
Visit
for acomplete selection.
tech
tip
Compatibility concerns?
Refer to the Septum SelectionGuide
at
TheUSP general chapter <467>Residual Solvents is awidely used compendial
method for identifying and quantifying residual solvents whenno information
is availableonwhat solvents are likely tobepresent. Inanattempt toharmonize
with the ICH guidelines, theUSPhas proposed amore comprehensivemethod
in thecurrentUSP30/NF25.This revision significantly increases thenumberof
residual solvents tobe routinely tested and includes three distinct procedures.
1
Initially set tobecome effective July 1, 2007, the implementationof the current
version of USP<467>has beendelayeduntil July 1, 2008. Until that time, the
Other Analytical Procedures section of the previous version will be retained.
However, in preparation for the implementation of the revised method, this
applicationwill complywith the procedure and criteria set forth in theUSP30/
NF25, second supplement (effectiveDecember1,2007)and the interim revision
announcement.
OverviewofMethod
The revisedUSP<467>method consists of a static headspace extraction cou-
pledwith a gas chromatographic separation and flame ionization detection. In
this guidewedemonstrate theUSP<467> applicationusing twodifferent types
of headspace autosamplers. ProcedureAwas performedusing a pressured loop
autosampler and transfer line. Procedure B was performed using a heated
syringe injection. Either system canbeused tomeetmethod requirements.
USP<467> is divided into two separate sections basedupon sample solubility:
water-soluble andwater-insoluble articles. Themethodology for both types of
articles is similar,but thediluentused inboth standardand samplepreparations
differs based upon the solubility of the test article. The testmethod consists of
three procedures (A, B, andC), that are designed to identify, confirm, and then
quantify residual solvents indrug substances andproducts (Figure 3).
1
This number of analytes to be tested represents the sum of Class 1 and 2 residual solvents that
can be effectively assayed usingHS/GC. The actual number of analytesmay bemore if xylenes,
ethyl benzene and
cis/trans
1,2 dichloroethylene are differentiated, or if circumstances require
the quantification of specific Class 3 residual solvents.
free
literature
Download your free copy of
ourTechnical Guide for Static
HeadspaceAnalysis from
lit. cat.# 59895A
including screw-threadheadspacevials
&magnetic screw-threadcaps!


