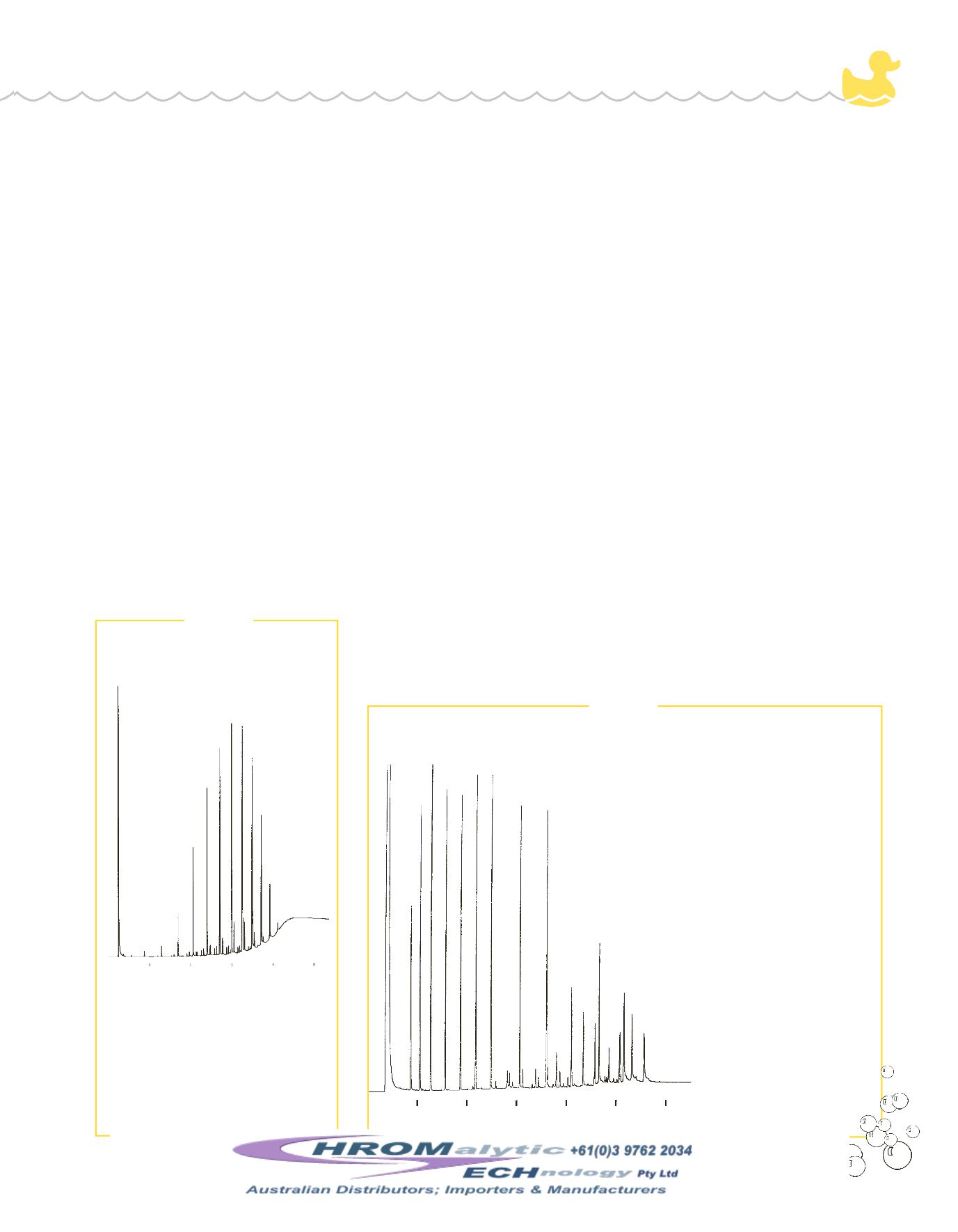
3
Figure 1
Triton
®
X-100 surfactant separated by
number of ethylene oxide units on an
MXT
®
-1 column.
Surfactant-containing solutions can be
applied to awide variety of surfaces,
including tile, ceramic, and cloth - and
hair. Builders often are used to increase
the effectiveness of a surfactant. Builders
reducewater hardness by “tying up”
hardnessminerals, through chelation
with theminerals or by forming an insol-
uble precipitate. Examples of
builders/chelating agents include sodium
citrate (the sodium salt of citric acid) and
ethylenediamine tetraacetic acid (EDTA).
Other builders, such as sodium carbon-
ate, reducewater hardness by forming
insoluble precipitates (e.g., calcium car-
bonate).
Surfactants generally are classified by
their ionic properties inwater.Anionic
surfactants, such as alcohol ethoxylates,
alkyl sulfates, and soaps, are negatively
charged in solution.Anionic surfactants
are used in laundry detergents and some
dishwashing detergents, household clean-
ers, and personal cleaning products.
Cationic surfactants, such as quaternary
ammonium compounds, carry a positive
charge in solution. They are used in
products such as fabric softeners.
Amphoteric surfactants, which can be
either positively or negatively charged,
often are used in personal cleansing
products, due to theirmildness.
Nonionic surfactants, such as alcohol
ethoxylates, are uncharged in solution;
they are used in laundry detergents and
automatic dishwasher detergents.An
example analysis of a nonionic surfac-
tant, Triton
®
X-100, an octylphenol eth-
ylene oxidewith an average of 9.5 ethyl-
ene oxide units permolecule, is shown
in Figure 1. This surfactant can be ana-
lyzed byGC, using a nonpolar phase,
such asMXT
®
-1.
As described above, soaps are anionic
surfactants. Basically, soaps are sodium
or potassium salts of fatty acids, pro-
duced by reacting animal or vegetable
fats or oilswith a strong alkali. The fat
or oil, in its original form, consists pri-
marily of triglycerides—three fatty acids
attached to a glycerol backbone.After
conversion to the soap—saponifica-
tion—there is both a hydrophilic (car-
min. 4
8
12
16
20
GC_CH00358
MXT
®
-1, 30m, 0.28mm ID, 0.10µm (cat.# 70109)
Inj.:
1.0µL split injection of Triton
®
X-100 (40:1) inmethylene
chloride
Oven temp.:
150°C to 400°C@15°C/min.
(hold 10min.)
Inj. / det. temp.: 250°C / 400°C
Carrier gas:
hydrogen
Linear velocity: 40cm/sec.
FID sensitivity: 102 x 10
-11
AFS
1
2
17
16
15
14
13
12
11
10
9
8
7 6
5 4
3
min. 4
8
12
16
20
24
Figure 2
Free fatty acids analysis saves time andmaterials, relative to preparing and analyzing
fatty acidmethyl esters.
GC_CH00283
1. C2:0 - acetic acid
2. C3:0 - propionic acid
3. C4:0 - butyric acid
4. C5:0 - valeric acid
5. C6:0 - caproic acid
6. C7:0 - heptanoic acid
7. C8:0 - caprylic acid
8. C10:0 - capric acid
9. C12:0 - lauric acid
10. C14:0 -myristic acid
11. C15:0 - pentadecanoic acid
12. C16:0 - palmitic acid
13. C16:1 - palmitoleic acid
14. C18:0 - stearic acid
15. C18:1 - oleic acid
16. C18:2 - linoleic acid
17. C18:3 - linolenic acid
Stabilwax
®
-DA, 30m, 0.53mm ID, 0.25µm (cat.# 11025)
Inj.:
0.5µL direct injection of a 5mg/mL standard
Oven temp.:
100°C (hold 2min.) to 250°C@8°C/min.
Inj. & det. temp.: 280°C
Carrier gas:
helium
Linear velocity: 40cm/sec. (flow rate: 5.2cc/min.)
FID sensitivity:
8 x 10
-11
AFS
boxylate group) and a hydrophobic end
(alkyl chain) to themolecule.Water, a
polarmolecule, can now interact with
the hydrophilic alkyl chains, while the
alkyl chain can interact with relatively
non-polar surfaces such as countertops,
tile, or skin.
Fatty acids that make up a soap can be
analyzed either in the free fatty acid
form or after derivatization to themethyl
esters (FAMEs). Figure 2 shows an
analysis of free fatty acids byGC, using
a Stabilwax
®
-DA capillary column. The
acid-deactivated phase in the Stabilwax
®
DA column gives excellent peak shapes
for free fatty acids. Figure 3 is an analy-
sis of fatty acids asmethyl esters, sepa-
rated on anRtx
®
-Wax column. FAMEs
also can be easily quantified by using a
Stabilwax
®
column.
Solvents
Solvents are used primarily to dissolve
organic soils. They also cleanwithout
leaving residue, making them very use-
ful in products such as glass cleaners.
Themain criterion for cleaning product
solvents iswatermiscibility, as the sol-
ventmust form a solutionwith the other
water-soluble components.Alcohols and
Excellent peak shapes
for free fatty acids
Website :
E-mail
TelNo : 03 9762 2034 . . . inAUSTRALIA


